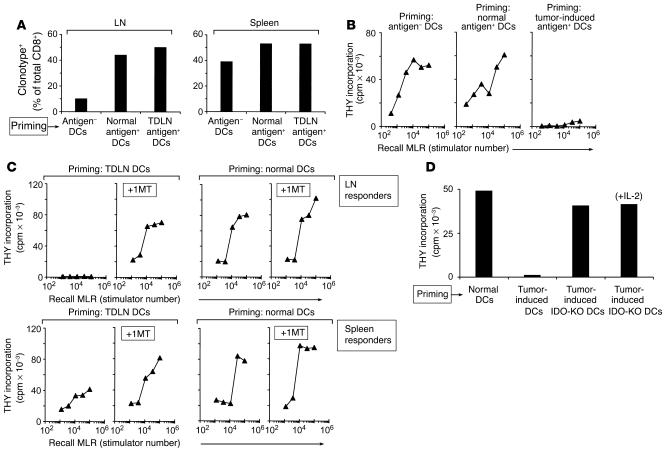
Figure 4.
Adoptive transfer of DCs from TDLNs creates immunosuppression in new hosts. (A) Recruitment of BM3 T cells to draining LNs. CD11c+ DCs were purified from TDLNs and injected subcutaneously into CBA mice; recipients had previously received 4 × 107 BM3 splenocytes intravenously (CBA+BM3 hosts). Control recipients received normal CD11c+ DCs from antigen-postive C57BL/6 mice without tumors (Normal antigen+ DCs); or normal DCs from antigen-negative CBA mice (Antigen– DCs). After 10 days, LNs draining the site of DC injection (left) and spleens (right) were harvested. BM3 cells were enumerated by FACS using anti-clonotypic antibody (expressed as a percentage of the total CD8+ T cells). Each bar represents four pooled nodes. (B) Functional unresponsiveness of T cells primed with DCs from TDLNs. CBA+BM3 mice were primed as described above, and LN cells were used as responders in recall MLRs (1 × 105 responder cells with a titration of irradiated C57BL/6 splenocyte stimulators). (C) CBA+BM3 mice were primed for 10 days with DCs from TDLNs (left) or normal C57BL/6 LNs (right). Half of each group received 1MT (5 mg/d) via subcutaneous pellet as described in Methods, from the time of adoptive transfer until the end of the experiment; the other half received vehicle alone. Recall MLRs were performed as above. (D) Creation of unresponsiveness required functional IDO in the transferred DCs. Tumors were grown in IDO-KO mice, and TDLN DCs were isolated and used to prime CBA+BM3 mice, as in the preceding panels. Control recipients received TDLN DCs from wild-type hosts, or normal DCs from non–tumor-bearing mice. Just as above, normal DCs did not create unresponsiveness in recall MLRs, and IDO-sufficient TDLN DCs created complete unresponsiveness. The IDO-KO DCs, even though from TDLNs, did not create unresponsiveness; and responses were not further enhanced by addition of exogenous IL-2 to the recall MLR (last bar), which argues against any component of partial or cryptic anergy.