Abstract
Placental hypoxia has been implicated in pregnancy pathologies, including fetal growth restriction and preeclampsia; however, the mechanism by which the trophoblast cell responds to hypoxia has not been adequately explored. Glucose transport, a process crucial to fetoplacental growth, is upregulated by hypoxia in a number of cell types. We investigated the effects of hypoxia on the regulation of trophoblast glucose transporter (GLUT) expression and activity in BeWo choriocarcinoma cells, a trophoblast cell model, and human placental villous tissue explants. GLUT1 expression in BeWo cells was upregulated by the hypoxia-inducing chemical agents desferroxamine and cobalt chloride. Reductions in oxygen tension resulted in dose-dependent increases in GLUT1 and GLUT3 expression. Exposure of cells to hypoxic conditions also resulted in an increase in transepithelial glucose transport. A role for hypoxia-inducible factor (HIF)-1 was suggested by the increase in HIF-1α as a result of hypoxia and by the increase in GLUT1 expression following treatment of BeWo with MG-132, a proteasomal inhibitor that increases HIF-1 levels. The function of HIF-1 was confirmed in experiments where the hypoxic upregulation of GLUT1 and GLUT3 was inhibited by antisense HIF-1α. In contrast to BeWo cells, hypoxia produced minimal increases in GLUT1 expression in explants; however, treatment with MG-132 did upregulate syncytial basal membrane GLUT1. Our results show that GLUTs are upregulated by hypoxia via a HIF-1-mediated pathway in trophoblast cells and suggest that the GLUT response to hypoxia in vivo will be determined not only by low oxygen tension but also by other factors that modulate HIF-1 levels.
Keywords: glucose transporter 1, glucose transporter 3, glucose transport
IT IS WIDELY BELIEVED that placental hypoxia (reduced oxygen tension) causes or contributes to pathologies such as intrauterine growth restriction and preeclampsia (6, 12, 15, 41, 45). Syncytiotrophoblasts, the specialized epithelial cells that comprise the barrier between the mother and fetus and form the site of nutrient and gas exchange, are known to be extremely sensitive to changes in oxygen tension early in placental development (14, 24, 31); however, trophoblast responses to hypoxia later in pregnancy are much less clear. One of the key proteins that responds to hypoxia in a variety of cells and species is the GLUT1 glucose transporter. GLUT1 is a member of the GLUT family, transmembrane proteins with (at least) 12 known isoforms that transport glucose by a sodium-independent, facilitated diffusion mechanism (30). GLUT1 is the only functionally relevant GLUT protein that has been identified in term human trophoblasts (26, 27). GLUT3 protein has been identified in first-trimester trophoblasts (46) as well as the BeWo choriocarcinoma cell line (23), which is frequently used as a model for the syncytiotrophoblast. A number of previous studies have examined GLUT1 expression in trophoblasts; GLUT1 in human trophoblast cells is regulated by glucose concentration (17, 18, 23, 36) and by glucocorticoids (16). In vivo, GLUT1 expression is modulated over gestation (27), in diabetic pregnancy (13), and by chronic hypoxia (56).
Although GLUT1 upregulation is a widely recognized response to hypoxia in multiple cell and tissue types, remarkably little is known about the response in trophoblast cells. Estermann et al. (9) showed that mRNA for both GLUT1 and GLUT3 was upregulated in primary syncytotrophoblast cells upon exposure to hypoxia (9), although glucose uptake was markedly reduced. Another more recent report (19) described increases in hypoxia-inducible factor (HIF)-1α and in GLUT1 mRNA and protein in trophoblast cells under hypoxic conditions. This research also demonstrated that the rat GLUT1 promoter, transfected into trophoblast cells, was activated by exogenous HIF-1. The goals of our study were to elucidate the effects of hypoxia on GLUT expression in BeWo choriocarcinoma cells and placental villous tissue fragments and to determine if regulation of endogenous GLUTs by hypoxia is mediated by HIF-1. The hypotheses tested were that, under hypoxic conditions, GLUT protein expression and activity are upregulated, that the levels of protein transporter expression correlate with the degree of hypoxia, and that regulation of GLUT expression under hypoxic conditions is mediated by the HIF-1 transcription factor.
MATERIALS AND METHODS
Materials
The BeWo choriocarcinoma cell line (b30 clone) was supplied by Dr. Kenneth Audus (University of Kansas). Antisense and sense phosphorothioate oligonucleotides were synthesized by the New Jersey Medical School Molecular Resource Facility. Polyclonal GLUT1 and GLUT3 antibodies were supplied by Chemicon (Temecula, CA) and the monoclonal anti-transferrin (TfR) antibody by Zymed (Invitrogen, Carlsbad, CA). The monoclonal β-actin antibody (AC74 clone), horseradish peroxidase-labeled secondary antibodies, and protease inhibitor mix (catalog no. 2714) were obtained from Sigma Chemical (St. Louis, MO). Permeable supports (PET; 0.9-cm diameter, 0.4-μm pore size) were obtained from Falcon (Becton Dickinson, Franklin Lakes, NJ). Nitrocellulose membranes were supplied by Bio-Rad (Hercules, CA), Sypro Ruby by Molecular Probes (Eugene, OR), Hyperfilm ECL by Amersham (Arlington, IL), and SuperSignal Chemiluminescence Kits by Pierce (Rockford, IL). The TransAm HIF-1α ELISA kit was obtained from Active Motif (Carlsbad, CA).
Cell culture
BeWo choriocarcinoma cells were plated in six-well plates and cultured in DMEM/F12–10% FBS until 80% confluent, as assessed by light microscopy. Prior to treatment, BeWo cells were serum starved in DMEM-5 mM glucose containing 0.5% BSA overnight. Different experimental approaches were used to mimic hypoxic conditions, including chemical hypoxia-inducing agents [cobalt and desferroxamine (DFO)] and a range of oxygen tensions. BeWo cells were incubated at 37°C in either the presence or absence of effectors (0.2 mM DFO and 0.2 mM cobalt chloride) or in a hypoxia chamber (Modular Incubator Chamber, MIC-101, Billups-Rothenberg, Del Mar, CA) gassed with 1%, 3%, or 5% oxygen. Following incubation, cells were washed with PBS and extracted at various time points ranging from 3 to 72 h using a lysis buffer containing 1% SDS, 20 mM HEPES (pH 7.4), and a protease inhibitor mixture. Under hypoxic conditions, proliferation was reduced compared with control cells; however, cell viability was not decreased. Even under severe hypoxia (e.g., 1% oxygen for 72 h), >98% of the cells excluded trypan blue, indicating cellular integrity.
In some experiments, transepithelial glucose transport was measured across a BeWo cell monolayer supported on a permeable membrane, as previously described (53). Briefly, BeWo cells were plated (~100,000 cells/well) and cultured on membrane inserts (0.4-μm pore size, 0.9-cm diameter). Cells were incubated in DMEM/ F-12 containing 10% FBS in both the upper and lower reservoirs. Monolayer confluence was assessed daily by measuring the transepithelial electrical resistance immediately following a medium change (Evohm, World Precision Instruments, Sarasota, FL). Experiments were performed 48 h after cells had been plated, when no additional change in transepithelial electrical resistance was found to occur. At this point, the medium was replaced by DMEM-5 mM glucose containing 0.5% BSA, and the wells were incubated in either 3% or 20% oxygen for 24 h. Following the 24-h incubation, at time 0, the medium in the lower reservoir was replaced with fresh glucose-free medium while the medium in the upper reservoir was replaced with DMEM-0.5% BSA containing 5 mM glucose. Cells were incubated on an orbital shaker (60 Hz) at room temperature. Samples from the lower reservoir were taken every 8 min over 40 min, and glucose concentrations were determined by a standard hexokinase-glucose-6-phosphatase assay (34). The rate of change of glucose concentration in the lower reservoir, which was linear over the measurement period, was calculated to give a measure of total glucose transport. Passive (i.e., non-carrier mediated) transport was determined by performing the same experiments in the presence of the glucose transport inhibitor phloretin (2 mM). The difference between these two measurements was taken as the rate of carrier-mediated glucose transport.
Apical and basal membrane fractions were prepared from BeWo cells using a magnesium precipitation technique. BeWo cells were cultured to near confluence in 100-mm culture plates, washed with warm HBSS, and then scraped off the plates using a cell scraper into 20 mM HEPES-Tris (pH 7.4) containing a protease inhibitor mix. The cell mixture was homogenized in a glass Dounce “A” homogenizer on ice for 10 strokes to break up the cells and vesiculate the membranes and then centrifuged at 3,000 g for 10 min. The pellet was discarded, and the supernatant was brought to 12 mM Mg2+ by the addition of MgCl2 and incubated on ice for 20 min. Following incubation, the homogenate was centrifuged at 3,000 g for 10 min. The supernatant was retained for the preparation of the apical membranes while the pellet containing the basal membrane fraction was resuspended in 20 mM HEPES-Tris (pH 7.4) containing 2 mM EDTA, 0.5% BSA, and protease inhibitor mix and frozen at −80°C. The supernatant was centrifuged at 75,000 g, and the resultant pellet, containing the apical membrane fraction, was resuspended in 20 mM HEPES-Tris (pH 7.4) containing protease inhibitor mix and frozen at −80°C.
Villous explants and syncytial membranes
Fresh placental tissue was obtained from elective cesarean deliveries (no labor) with the approval of the New Jersey Medical School Institutional Review Board using written informed consent. Villous explants were prepared by washing pieces of core villous tissue with cold 0.9% saline and then dissecting the tissue to produce 2- to 5-mm villous explant samples. The explant samples (2–4 g) were incubated in DMEM-5 mM glucose containing 0.5% BSA in hypoxia chambers containing 3% or 20% oxygen at 37°C. Following incubation, explant samples were blotted dry and homogenized in 250 mM sucrose and 10 mM HEPES-Tris (pH 7.4) containing protease inhibitor mix. The homogenate was centrifuged at 10,000 g for 15 min, and the supernatant was retained, brought to 12 mM MgCl2, and incubated on ice for 20 min. After incubation, the mixture was centrifuged at 2,500 g for 10 min. The pellet was resuspended in 250 mM sucrose and 10 mM HEPES-Tris (pH 7.4) containing 2 mM EDTA, 0.5% BSA, and protease inhibitor mix to produce the basal membrane-enriched fraction (BMF), while the supernatant was removed and centrifuged at 75,000 g for 30 min and then resuspended in 250 mM sucrose and 10 mM HEPES-Tris (pH 7.4) containing protease inhibitor mix to produce the microvillous fraction (MVF).
Antisense experiments
BeWo cells were incubated with 15-bp phosphorothioate sense or antisense HIF-1α oligonucleotides in the absence or presence of different hypoxic stimuli. The sequences used for the sense and antisense oligonucleotides were obtained from previously published data in which these oligonucleotides were used to block HIF-1α expression in first-trimester trophoblasts (5). Transporter expression is presented in the RESULTS and figures as the fractional change relative to a control sample consisting of cells exposed to 20% oxygen and sense oligonucleotide.
Immunoblot analysis
Whole cell or syncytial membrane extracts (5 μg protein) were slot blotted on nitrocellulose membranes according to the manufacturer’s protocol (Biodot SF, Bio-Rad). To correct for differences in protein loading, the quantity of protein loaded was determined using a fluorescent technique; nitrocellulose membranes were incubated for 15 min in 7% acetic acid and 10% methanol, washed with distilled water, and incubated with Sypro Ruby fluorescent stain for 15 min. Membranes were washed extensively in distilled water, and signals were digitized on a Typhoon 9410 imager (GE Healthcare BioScience, Piscataway, NJ). GLUT1 or GLUT3 expression was evaluated using specific polyclonal antibodies that have been previously shown to yield a single band on Western blot. Specificity was assured by absence of a signal following omission of the primary antibody. Membranes were blocked with 3% BSA in Tris-buffered saline (TBS) for 60 min and then incubated with rabbit polyclonal anti-GLUT1 or GLUT3 antibodies (1:20,000) or mouse monoclonal anti-TfR or anti-β-actin antibodies (1:10,000) in TBS containing 0.5% BSA for another 60 min. After membranes were washed with TBS containing 0.05% Tween 20 (TBST; 1 × 15 min and 2 × 10 min), a secondary antibody was applied (horseradish peroxidas-coupled goat anti-rabbit or anti-mouse IgG, 1:50,000) for 60 min. After membranes were washed with TBST (1 × 15 min and 2 × 10 min), immunore-active bands were visualized by chemiluminescence and digitized on a Typhoon 9410 imager. All the preceding steps were performed at room temperature. Slot density was quantitated by densitometry (ImageJ, National Institutes of Health), and the intensity of the signal obtained on the immunoblot was normalized using the protein loading values obtained from the Sypro Ruby measurements. In addition to the experimental samples loaded on to the blots, a series of reference standards were loaded on to each blot, comprising a pooled set of samples. The reference sample loading provided a range from 1 to 15 μg of protein, encompassing the sample loading to ensure that the experimental sample densities were within the linear range.
Statistics
Data are given in the figures and RESULTS as means ± SE. The number (n) given in the RESULTS and figures represents the number of experiments. For each experiment using BeWo cells, each point was performed in triplicate, i.e., three different wells per point were collected. When two conditions were compared, Student’s t-test was used, whereas for multiple experimental conditions, one-way ANOVA was employed. Differences were taken as significant when P < 0.05.
Other
Assays for HIF-1α were performed using the TransAm ELISA kit according to the manufacturer’s instructions (Active Motif, Carlsbad, CA). Alkaline phosphatase assays were performed using p-nitrophenol as a substrate (4). Protein assays were performed using the Bio-Rad Quick Start Protein Assay.
RESULTS
Regulation of GLUT1 expression by hypoxic stimuli
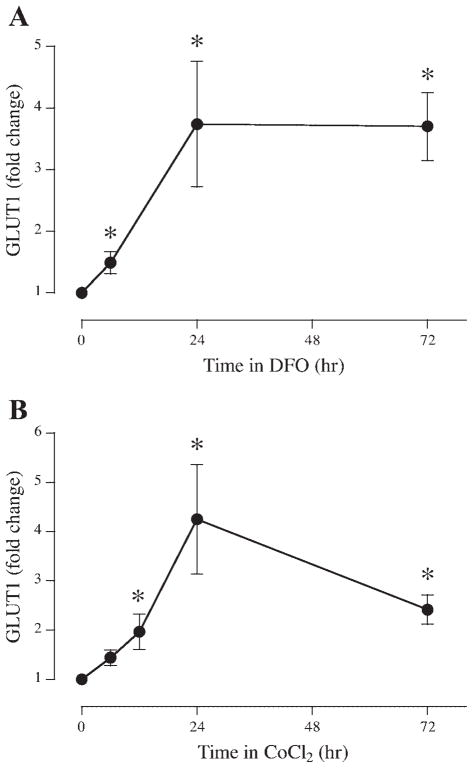
To investigate the trophoblast response to a hypoxic stimulus, serum-starved BeWo cells were incubated in DMEM-5 mM glucose containing 0.5% BSA in the absence or presence of an iron chelating agent, DFO (0.2 mM). GLUT1 expression was increased 1.49 ± 0.18-, 3.74 ± 1.02-, and 3.70 ± 0.55-fold at 6, 24 and 72 h compared with untreated cells (Fig. 1A; P < 0.05, n = 6).
Fig. 1.
Time course of the effects of hypoxic agents on BeWo glucose transporter 1 (GLUT1). Serum-starved BeWo cells were incubated in 0.2 mM desferroxamine (DFO; A) or 0.2 mM cobalt chloride (B) for up to 72 h (n = 6). At the time points noted, samples were extracted, analyzed for GLUT1 expression by slot immunoblotting, and compared with timed, untreated control incubations. *P < 0.05 vs. control.
To determine whether the same pattern of GLUT1 expression could be observed after exposure to a different hypoxic stimulus, BeWo cells were exposed to cobalt chloride (0.2 mM). After a 6-h exposure, GLUT1 expression was not altered (Fig. 1B); however, expression was increased at 12 h by 1.97 ± 0.36-fold compared with untreated cells. Further upregulation of GLUT1 expression was observed after 24 h (4.25 ± 0.11) and 72 h (2.42 ± 0.30) of cobalt exposure (P < 0.05, n = 6).
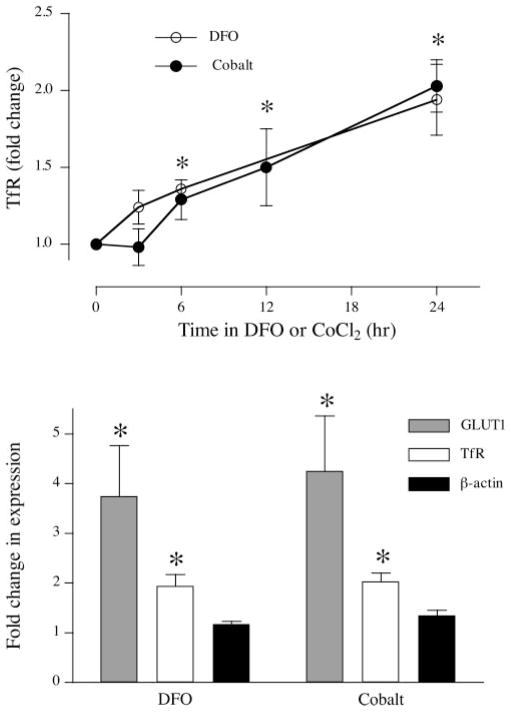
Response of TfR and β-actin to hypoxic stimuli
To compare the response of hypoxia-responsive and hypoxia-insensitive proteins in BeWo cells, we measured the expression of TfR and β-actin. TfR expression over time was measured following incubation with DFO or cobalt as described above (Fig. 2A). The results show that TfR was upregulated by both DFO and cobalt in a time-dependent manner (P < 0.05, n = 4). Because both GLUT1 and TfR are predicted as being responsive to hypoxic, it was important to demonstrate the specific nature of these changes. We therefore compared the changes in GLUT1 and TfR observed after 24 h in DFO or cobalt with the expression of β-actin, a structural protein that does not respond to hypoxia. Figure 2B shows that while there was significant upregulation of both GLUT1 and TfR, β-actin expression was not affected by DFO [1.17 ± 0.06, not significant (NS), n = 3] or by cobalt (1.35 ± 0.10, NS, n = 3).
Fig. 2.
Effect of hypoxic agents on BeWo markers of hypoxia. A: serum-starved BeWo cells were incubated in 0.2 mM DFO or 0.2 mM cobalt chloride for up to 24 h (n = 4). At the time points noted, samples were extracted and analyzed for transferrin receptor (TfR) expression compared with untreated control samples. B: serum-starved BeWo cells were incubated in 0.2 mM DFO or 0.2 mM cobalt chloride for 24 h. Samples were extracted, analyzed for GLUT1 (n = 6), TfR (n = 4), and β-actin (n = 3) expression by slot immunoblotting, and compared with untreated controls. *P < 0.05 vs. control.
Regulation of GLUT1 expression by low oxygen tension
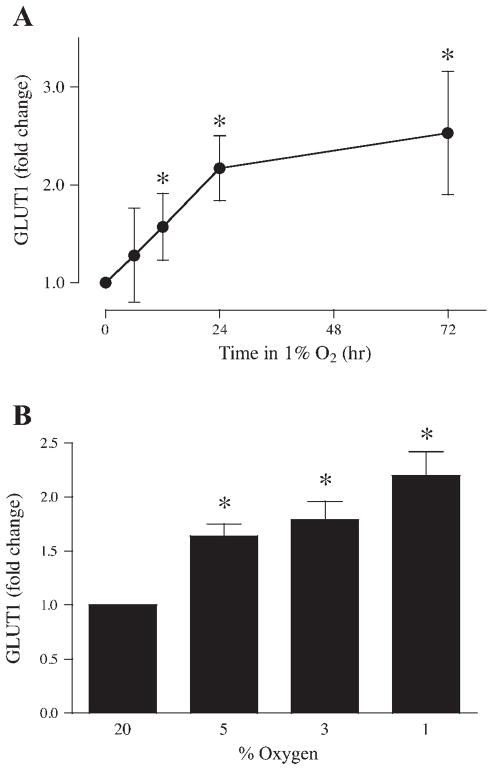
To analyze the response of GLUT1 expression to low oxygen tension, serum-starved BeWo cells were incubated in DMEM-5 mM glucose containing 0.5% BSA and exposed to atmospheres of 1% or 20% (control) oxygen. Cells were extracted after 6, 12, 24, and 72 h and analyzed for GLUT1 expression by slot blotting. After 6 h of exposure to 1% oxygen, GLUT1 expression was not increased compared with control (Fig. 3A); however, GLUT1 expression increased progressively to 1.57 ± 0.34-, 2.17 ± 0.33-, and 2.53 ± 0.63-fold of control after 12, 24, and 72 h, respectively (P < 0.05, n = 6).
Fig. 3.
Effects of reduced oxygen tension on BeWo GLUT1 expression. A: time course of GLUT1 expression for serum-starved BeWo cells incubated in 1% oxygen for up to 72 h (n = 6). B: oxygen dependence of GLUT1 expression in BeWo cells incubated for 24 h in 1%, 3%, 5%, or 20% oxygen (n = 6). *P < 0.05 vs. samples incubated in 20% oxygen.
Experiments were also performed with different levels of oxygen to determine whether there was a dose response for the effect of oxygen on GLUT1 expression. Following incubation in 1%, 3%, 5%, or 20% oxygen for 24 h, cells were extracted, and GLUT1 protein was measured in lysates by slot immunoblotting. Incubation of the cells at decreasing levels of oxygen increased the expression of GLUT1 progressively from a 1.64 ± 0.11-fold increase at 5% oxygen to 1.79 ± 0.17-fold at 3% oxygen and to 2.20 ± 0.22-fold at 1% oxygen (Fig. 3B; P < 0.05, n = 6).
GLUT3 and hypoxia
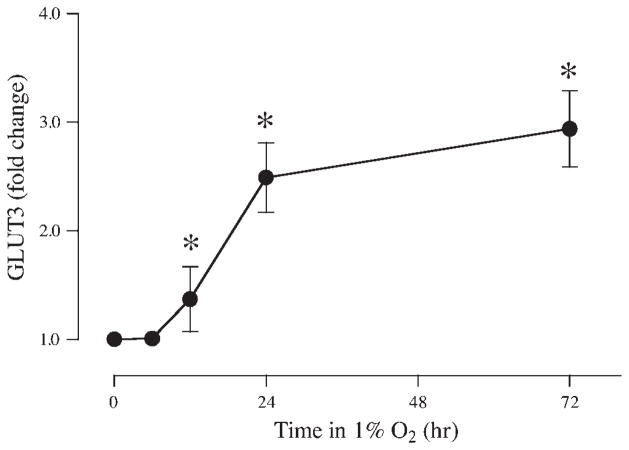
Although GLUT1 is the only GLUT family isoform present in the syncytium at term (26, 27), GLUT3 is present in first-trimester trophoblasts (43) and also in BeWo cells (23, 43), which are derived from first-trimester tissue. To evaluate whether GLUT3 was impacted by hypoxia in a manner similar to GLUT1, the expression of GLUT3 was measured after exposure to 1% oxygen at 6, 12, 24, and 72 h. No difference was observed after 6 h (Fig. 4), but after 12, 24, and 72 h in 1% oxygen, GLUT3 was increased by 1.37 ± 0.30-, 2.49 ± 0.32-, and 2.94 ± 0.35-fold, respectively, compared with control cells incubated in 20% oxygen (P < 0.05, n = 4). Thus, GLUT3, like GLUT1, appears to be upregulated by hypoxia in a time-dependent manner.
Fig. 4.
Time course of the effects of reduced oxygen tension on BeWo GLUT3 expression. Serum-starved BeWo cells were incubated in 1% or 20% oxygen for up to 72 h. At the time points noted, samples were extracted and analyzed for GLUT3 expression (n = 4). *P < 0.05 vs. samples incubated in 20% oxygen.
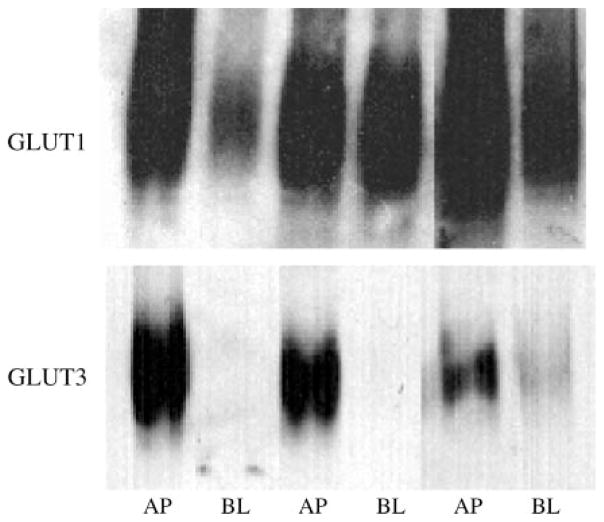
Although GLUT3 has been described previously in BeWo and other choriocarcinoma cells, the cellular localization (apical/basal) has not been determined. To localize GLUT1 and GLUT3 in BeWo cells, we performed a preparative procedure to isolate apical and basal membrane fractions using magnesium to precipitate the basal membrane fraction. The apical marker alkaline phosphatase was enriched by 11.3 ± 3.2-fold in the apical membrane fraction compared with cell homogenates, whereas the basal membrane fraction showed a 1.1 ± 0.1-fold enrichment (n = 7), demonstrating separation of the two fractions. The two fractions were Western blotted for GLUT1 and GLUT3 using specific polyclonal antibodies (Fig. 5). As Fig. 5 shows, GLUT1 was detected in both the apical and basal fractions; however, GLUT3 was detected only in the apical fraction. These data show that GLUT3 is localized to the apical membrane of BeWo cells.
Fig. 5.
Localization of GLUT1 and GLUT3 in BeWo cells. BeWo apical (AP) and basal (BL) membrane fractions were isolated using magnesium precipitation, extracted, and then separated by SDS-PAGE. After transfer, membranes were blotted for GLUT1 and GLUT3. Adjacent AP and BL samples were obtained from the same cell samples.
Hypoxia and glucose transport
Although hypoxia increases the expression of cellular GLUT1, it is important to determine whether the increase is physiologically relevant in terms of glucose transport. The extent to which upregulation of GLUT expression leads to increased transport activity was investigated by measuring transepithelial glucose transport following incubation under normoxic or hypoxic conditions. BeWo monolayers, grown to confluence on permeable supports, were exposed to 3% or 20% oxygen for 24 h. Transepithelial transport was assessed by measuring the concentration of glucose in the lower (initially glucose free) reservoir following the addition of medium containing 5 mM glucose to the upper reservoir. Glucose transport was determined in the presence or absence of 2 mM phloretin, a specific inhibitor of carrier-mediated glucose transport. The transport measurement was taken as the difference between the transfer measured in the presence and absence of phloretin. After a 24-h exposure to 3% oxygen, carrier-mediated transepithelial glucose transport was 1.23 ± 0.13 nmol·min−1 ·well−1 compared with cells incubated in 20% oxygen, which had a transport rate of 0.81 ± 0.07 nmol·min−1·well−1 (P < 0.05, n = 5), i.e., hypoxia increased carrier-mediated glucose transport across the BeWo cell monolayer by 1.54 ± 0.16-fold.
Regulation of GLUT1 expression during hypoxic stress
There is substantial evidence to suggest that GLUT1 responses to hypoxia are modulated by the transcriptional regulator HIF-1. We therefore measured the expression of GLUT1 after treatment of cells with the proteasome inhibitor MG-132, an agent that inhibits proteasomal degradation of ubiquitinylated proteins, including HIF-1α, thus increasing the intracellular level of HIF-1. Following incubation of BeWo cells for 6 h under normoxic conditions in the presence of 10 μM MG-132, cell extracts were assayed for GLUT1. The expression of GLUT1 was increased 1.68 ± 0.12-fold as a result of treatment with MG-132 (P < 0.05, n = 4). These results suggest that GLUT1 expression may be regulated by HIF-1.
To confirm that the upregulation of GLUT1 in response to hypoxia was associated with a concomitant increase in HIF-1, we measured the HIF-1 α-subunit in normoxic and hypoxic cells using an ELISA for the HIF-1 α-subunit. HIF-1α was measured in nuclear extracts from BeWo cells following a 24-h exposure to 1% or 20% oxygen. The HIF-1α concentration was below the limit of detection (equivalent to 2.5 μg of the reference nuclear extract) in BeWo cell extracts following incubation under control conditions (20% oxygen). In cells exposed to 1% oxygen, however, HIF-1α was clearly detectable (equivalent to 52 ± 15 μg of the reference nuclear extract, n = 3), demonstrating the upregulation of HIF-1α by ≥20-fold.
GLUT1 expression and HIF-1
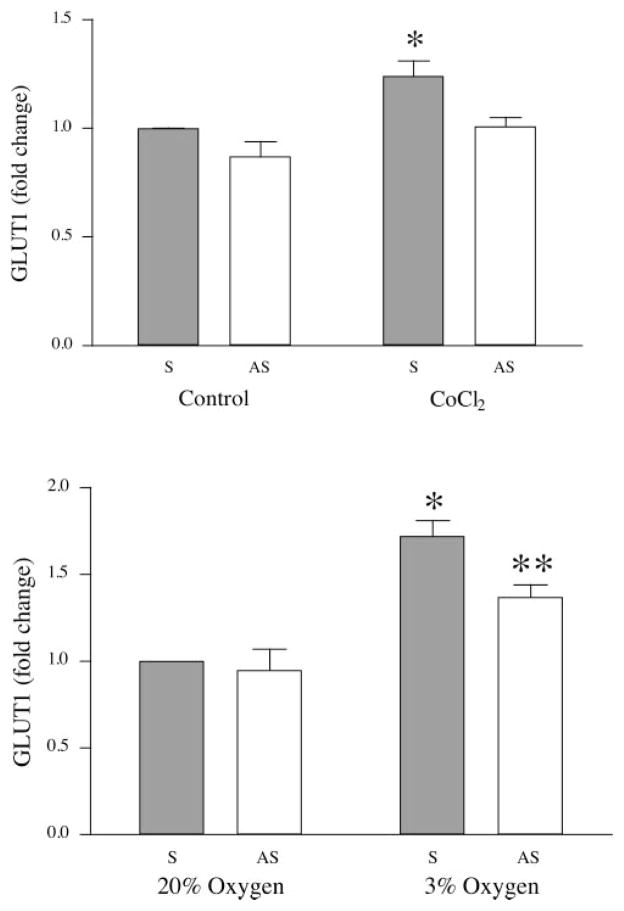
To confirm a causal relationship between HIF-1 and GLUT1 expression, an antisense oligonucleotide to HIF-1α was employed to block HIF-mediated expression of GLUT1 using a chemical agent (cobalt chloride) to induce hypoxia. The antisense oligonucleotide used here was used previously to block HIF-1α expression in trophoblast cells (5). BeWo cells were incubated with sense or antisense HIF-1α oligonucleotide (5 μM) before and during the period of hypoxia. Cells were preincubated for 6 h in the presence of sense or antisense oligonucleotide and then incubated in the absence or presence of 0.2 mM cobalt chloride for another 6 h. The oligonucleotide remained in the incubations during the hypoxic treatment period. GLUT1 expression is shown as the percent change relative to cells exposed to 20% oxygen and sense oligonucleotides (control). After a 6-h exposure to cobalt, GLUT1 expression was increased by 1.34 ± 0.07-fold (Fig. 6A; P < 0.05, n = 4). This upregulation was completely inhibited by an incubation with the antisense oligonucleotide.
Fig. 6.
Effects of sense (S) and antisense (AS) oligonucleotides on GLUT1 expression. A: serum-starved BeWo cells were incubated in sense or antisense oligonucleotides for 6 h, at which time cells were divided into groups and treated with 0.2 mM cobalt chloride or vehicle (n = 4). B: serum-starved BeWo cells were incubated in sense or antisense oligonucleotides for 6 h, at which time cells were divided into groups and incubated in 3% or 20% oxygen for a further 18 h (n = 4). *P < 0.05 vs. sense oligonucleotide-treated control; ** P < 0.05, sense oligonucleotide-treated control compared with sense oligonucleotide-treated experimental sample (3%).
A second set of experiments was designed to study the effects of antisense HIF-1α during exposure to reduced oxygen tension. Cells were incubated in sense or antisense oligonucleotide for 6 h under normoxic conditions and then divided into groups exposed to 20% or 3% oxygen for a further 18 h. For cells incubated with sense oligonucleotide, GLUT1 expression was 1.72 ± 0.09-fold higher following exposure to 3% oxygen compared with those samples exposed to 20% oxygen (Fig. 6B; P < 0.05, n = 4). Incubation with the antisense oligonucleotide partially attenuated the GLUT1 response (1.37 ± 0.07-fold elevation vs. control, P < 0.05, n = 4). No differences in GLUT1 expression were observed between samples treated with sense or antisense oligonucleotides following exposure to 20% oxygen (0.95 ± 0.12, NS, n = 4), excluding a toxic effect of antisense nucleotides, and demonstrating that the basal level of GLUT1 expression is independent of HIF-1α.
GLUT3 expression and HIF-1
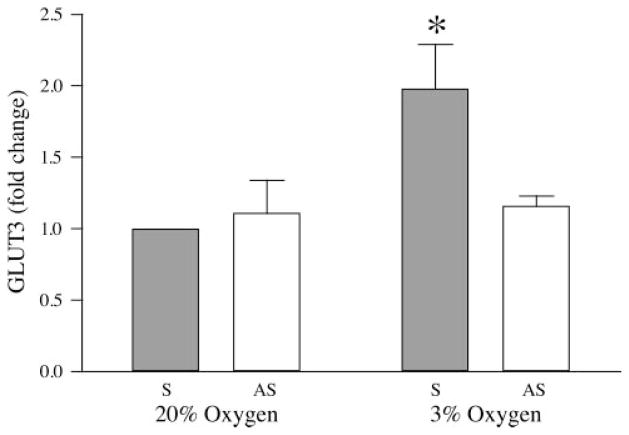
To examine the regulation of GLUT3 expression in BeWo choriocarinoma cells, GLUT1 experiments using specific oligonucleotides to block the HIF-1 pathway were repeated for the measurement of GLUT3. BeWo cells were incubated with either sense or antisense HIF-1α oligonucleotides for a total of 24 h, including exposure to 3% or 20% oxygen for the last 18 h. Following exposure to 3% oxygen, GLUT3 expression was upregulated by 1.98 ± 0.31-fold compared with sense-treated cells exposed to 20% oxygen (Fig. 7; P < 0.05, n = 4). This increase was completely inhibited following treatment with antisense oligonucleotide (1.16 ± 0.07 of control, NS, n = 4). No differences in GLUT3 expression were observed between sense and antisense treated samples exposed to 20% oxygen (1.11 ± 0.23, NS, n = 4).
Fig. 7.
Effects of sense and antisense oligonucleotides on GLUT3 expression stimulated by reduced oxygen tension. Serum-starved BeWo cells were incubated in sense or antisense oligonucleotides for 6 h, at which time cells were divided into groups and incubated in 3% or 20% oxygen for a further 18 h (n = 4). *P < 0.05 vs. sense-treated control.
Hypoxia in placental explants
To investigate the GLUT1 response to lowered oxygen tension in syncytiotrophoblast cells, villous tissue fragments were exposed to 3% or 20% oxygen for 6 h, and, following incubation, MVF and BMF were prepared and assayed for GLUT1. After a 6-h incubation, GLUT1 expression in the hypoxic samples showed no changes in either the BMF or MVF relative to samples incubated in 20% oxygen, similar to the 6-h results observed in BeWo cells (see above). We therefore tested the effects of MG-132, a proteasomal inhibitor, on GLUT1 expression. Villous tissue fragments were incubated for 6 h in the presence or absence of 10 μM MG-132 under normoxic conditions (20% oxygen). In the MVF, no difference in GLUT1 expression was seen between control and MG-132-treated samples (1.19 ± 0.10-fold of control, NS, n = 4); however, in the BMF, treatment with MG-132 increased GLUT1 expression by 1.42 ± 0.05-fold (P < 0.05, n = 4), similar to the results shown for BeWo cells.
DISCUSSION
In this study, we explored the response of the trophoblast GLUT family members to hypoxic stimuli in BeWo choriocarcinoma cells and human placental explants, hypothesizing that GLUT protein expression would be upregulated in a dose-dependent, HIF-1-mediated manner. Experiments performed at low oxygen tension produced a substantial and extended upregulation of GLUT1 expression, increasing in a time- and dose-dependent manner. GLUT3 expression in BeWo was also upregulated by hypoxic conditions. The changes in GLUT expression under hypoxic conditions were functional, as we demonstrated an increase in transepithelial glucose transport across a BeWo monolayer exposed to hypoxia. Mediation of the hypoxic response by the transcription factor HIF-1 was suggested both by an increase in HIF-1α in hypoxia and by an increase in GLUT1 expression following treatment of normoxic BeWo cells with the proteasome inhibitor MG-132. The involvement of HIF-1 was confirmed by demonstrating the effect of a HIF-1α antisense oligonucleotide in preventing the hypoxic upregulation of GLUT1 and GLUT3. In contrast to the results observed in BeWo cells, however, there was little or no increase in the expression of GLUT1 in syncytial membranes from placental explants as a result of hypoxia, although inhibition of HIF-1α degradation did enhance GLUT1 expression.
BeWo cells are a human trophoblast-derived choriocarcinoma line that demonstrates great similarities with primary trophoblast cells, and thus this line has been used extensively as a trophoblast model (3, 37). BeWo cells are similar to primary trophoblast in their morphology (22) and in their invasive nature (21, 25, 38). Like primary trophoblast cells, BeWo cells also undergo syncytialization (1, 54, 55), forming a well-differentiated monolayer. Syncytialization leads to a spectrum of changes that resemble those observed in the primary syncytiotrophoblast. These include changes in the expression of proteins involved in the syncytialization process, such as syncytin and the amino acid transporter, ASCT2 (32, 33), as well as alteration in the complement and expression of amino acid transporters (11, 40) and secretion of proteins such as human chorionic gonadotropin. There are also similarities in the expression and activity of a large number of other proteins including receptors and transporters which have been well described in the literature (2, 7, 8, 10, 29, 35, 39, 50). Thus, while there are also some differences between BeWo and primary trophoblast, such as the expression of GLUT3 in BeWo and the absence of the sodium-coupled neutral amino acid transporter, SNAT4 (42), BeWo cells nevertheless provide a useful and stable trophoblast model.
For certain aspects of experimentation, BeWo cells have clear advantages over primary trophoblast cells. First among these is the stability of the model. Primary trophoblast cells in culture undergo a continuous transition from primary cytotrophoblast to the full syncytium, and investigation of the effects of hypoxia on GLUTs must be distinguished from the effects of hypoxia on the aggregation, fusion, and differentiation processes. Use of BeWo cells allowed us to examine GLUT responses to hypoxia separate from the fundamental process of syncytial development. Another advantage to the BeWo cell is the ability to use a monolayer system to investigate changes in transepithelial glucose transport, a process that is not amenable to investigation in primary trophoblast cultures. As noted below, the ability to measure transepithelial transport allowed us to draw conclusions about changes in the transport process. The use of a robust trophoblast model also allowed us to use relatively high levels of antisense oligonucleotide to knock down HIF-1α expression in the absence of cytotoxicity.
The use of placental villous explants in this study is problematic. There is literature evidence of syncytiotrophoblast degeneration in explants incubated beyond 6 h (44, 48, 49). Nevertheless, we were able to obtain valuable information from villous explants over a 6-h period. While there were no changes in GLUT1 expression in the MVF or BMF as a result of 6 h of hypoxia, a similar length of incubation with MG-132 produced a significant increase in GLUT1 in the BMF, suggesting a rapid response to HIF-1α-mediated changes in villous tissue.
The response of GLUT1 protein expression in BeWo cells noted here is similar to that observed by Esterman et al. (9) in primary cultured syncytial cells, in which they observed increases in both GLUT1 and GLUT3 mRNA in response to hypoxia. Our findings of increased GLUT1 protein expression are consistent with the increases in mRNA for these transporters described by Esterman et al. (9). Increased GLUT1 mRNA and protein were also observed in BeWo cells by Hayashi et al. (19) following treatment with 5% oxygen or 0.25 mM cobalt. We tested the specificity of the GLUT1 response to hypoxia by measuring, in the same samples, the expression of TfR, another protein predicted to be upregulated by hypoxia, and β-actin, a structural protein that does not respond to decreased oxygen tension. While TfR expression was increased by both DFO and cobalt in BeWo cells, β-actin expression was unaffected, demonstrating the specificity of the response. DFO and cobalt are hypoxia mimetics that induce HIF-1α stabilization in normoxic conditions. DFO appears to act by chelation effects on the iron-dependent prolyl hydroxylases that are involved in oxygen sensing and the HIF-1α degradation pathway (52); however, the mechanism of cobalt has not been elucidated. Nevertheless, the correspondence between hypoxia and the hypoxia mimetics demonstrates that the effects of low oxygen levels were not due to diminished energy supply, since the substrate for oxidative metabolism was unaltered in the DFO/ cobalt experiments.
Beyond the upregulation of GLUT protein expression is the question of whether such increases are functional. After a 24-h exposure to 3% oxygen, glucose transport across the BeWo monolayer was 1.5 times higher than transport across the normoxic control. This increase in glucose transport is unlikely to result from decreased metabolism or an increase in synthesis, since previous data have shown that transepithelial glucose transport in BeWo cells is reduced, not increased, as a result of decreased oxidative metabolism (53). These observations show that low oxygen tension leads not only to oxygen-mediated upregulation of GLUT expression but also to an increased glucose transport activity. The glucose transport data reported here, showing increased glucose transport activity across the BeWo monolayer following hypoxia, contrasts with the data of Esterman et al. (9), in which hypoxic conditions caused a decrease in cellular glucose uptake; however, our data show similarity to those reported by Hayashi et al. (19), who observed a 150% increase after 24 h of incubation with 0.25 mM cobalt chloride. The notable difference between the data reported here and those reported previously (9, 19) is that prior work reported total cellular uptake of glucose, whereas this report describes measurements of transepithelial glucose transport. In light of previous data reported by this laboratory (53) demonstrating that the BeWo cell basal membrane is the rate-limiting step in transepithelial transport, we conclude that the increase in transport following hypoxic exposure is due to an increase in basal membrane GLUT expression. The localization data show that only GLUT1 is present in the basal membrane; thus, the change in transepithelial transport appears to be due to an increase in the expression of GLUT1 on the basal membrane. This does not rule out increases in apical GLUT1 and GLUT3 but demonstrates that the functionally important change is an increase in basal GLUT1 expression. The observation that MG-132 stimulated an increase in explant GLUT1 expression in the BMF but not the MVF strongly supports the conclusion that processes similar to those in BeWo cell are occurring in the villous syncytiotrophoblast.
In other tissues, under hypoxic conditions, expression of HIF-1 is increased, resulting in the upregulation of a variety genes associated with the response to hypoxia, including GLUTs (20, 47). It was hypothesized that trophoblast GLUT1 would also be regulated through the HIF-1 pathway. Initial experiments demonstrated that while HIF-1α protein was not detectable in BeWo cells exposed to 20% oxygen, HIF-1α was clearly detectable following exposure to 1% oxygen. Treatment of cells with antisense HIF-1α oligonucleotide caused no change in GLUT1 expression under normoxic conditions, demonstrating that HIF-1 appears not to be involved in the regulation of basal GLUT1 expression under normoxic conditions, and consistent with the absence of detectable HIF-1α observed in normoxic cultures. Hypoxia-mediated upregulation of GLUT1 expression was only partially inhibited by antisense oligonucleotide, suggesting either that a fraction of the GLUT1 upregulation is not mediated via HIF-1 or that the antisense oligonucleotide at the concentration used here (5 μM) is insufficient to block the stimulus generated by exposure to 3% oxygen (higher levels of nucleotides were not used because of the cytotoxic potential at these concentrations).
Another technique used to probe the involvement of HIF-1 in hypoxia-mediated regulation of GLUT1 was the effect of the proteasomal inhibitor MG-132. Under normoxic conditions, MG-132 treatment upregulated GLUT1 expression in BeWo cells and in the syncytial BMF of villous explants compared with their respective untreated controls. In fact, upregulation by MG-132 was more effective in the short term (6 h) than exposure to hypoxic conditions in both BeWo and explants, possibly because its effect on HIF-1α is relatively quick, whereas exposure to reduced oxygen tension may take longer to be effective. These findings are consistent with the involvement of the HIF-1 pathway in the regulation of GLUT1 expression in BeWo cells and in syncytial cells from villous tissue. Our data suggesting involvement of the HIF-1 pathway complement the investigation by Hayashi et al. (19), who showed that 0.25 mM cobalt or 5% oxygen exposure increased both GLUT1 expression and HIF-1α levels in BeWo cells. They also demonstrated the activation of a rat GLUT1 promoter construct containing a hypoxia-response element, when transfected into BeWo cells, by both 0.25 mM cobalt and exogenous HIF-1α. Our data show that the regulation of endogenous GLUT1 expression is modulated via the HIF-1 pathway present in BeWo cells.
The expression pattern of GLUT3, found in BeWo cells, was investigated in a manner similar to GLUT1. GLUT3 expression responded to low oxygen levels in a time-dependent manner. GLUT3 expression was analyzed following a hypoxic stimulus in the presence and absence of the HIF-1α antisense oligonucleotide. Following 24 of h exposure to 3% oxygen, GLUT3 in BeWo cells was markedly upregulated, and this was completely inhibited by antisense oligonucleotide, demonstrating that the HIF-1 pathway plays a role in oxygen-mediated regulation of GLUT3. The partial attenuation by antisense oligonucleotide of the GLUT1 response to hypoxia and the limited response of GLUT1 in villous explants suggests that other factors may function in villous tissue to modulate the GLUT1 response.
The nature of the hypoxic response mechanism is important because we have previously shown that in chronic hypoxic exposure in vivo, despite the changes in expression of markers of hypoxia such as the erythropoietin receptor and TfR, there is downregulation of basal membrane GLUT1 expression (56), contrary to the present results. The fact we have shown here, that the response to hypoxia is mediated by HIF-1, means that in vivo, the transporter response to reduced oxygen tension will be modified by an interaction with other regulatory pathways mediated via HIF-1. Most importantly, insulin and IGFs operate via HIF-1 (51, 57), and thus the in vivo response to hypoxia will depend on the interaction of (at least) the hypoxia and insulin/IGF mechanisms. In vivo, acute hypoxia will induce the upregulation response observed in our cell system, well prior to effects on growth mediated through decreased insulin and IGF secretion. The response of syncytiotrophoblast GLUTs to chronic hypoxia, however, is likely to be a net response that combines upregulation due to hypoxia with downregulation due to decreased growth factor action. This interaction may explain the lack of change in GLUT expression that has been observed thus far in fetal growth restriction (27, 28). Future research should thus be aimed at determining the means by which trophoblast cells integrate and respond to multiple, simultaneous extracellular signaling events including alterations in nutrient levels, changes in growth factor concentrations, and hypoxia.
Acknowledgments
The authors thank the staff of Labor and Delivery at the University of Medicine and Dentristry of New Jersey-University Hospital for the help in obtaining placental tissue.
GRANTS
This research was funded in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-55369.
References
- 1.Al-Nasiry S, Spitz B, Hanssens M, Luyten C, Pijnenborg R. Differential effects of inducers of syncytialization and apoptosis on BeWo and JEG-3 choriocarcinoma cells. Hum Reprod. 2006;21:193–201. doi: 10.1093/humrep/dei272. [DOI] [PubMed] [Google Scholar]
- 2.Aplin JD. Expression of integrin alpha 6 beta 4 in human trophoblast and its loss from extravillous cells. Placenta. 1993;14:203–215. doi: 10.1016/s0143-4004(05)80261-9. [DOI] [PubMed] [Google Scholar]
- 3.Bode CJ, Jin H, Rytting E, Silverstein PS, Young AM, Audus KL. In vitro models for studying trophoblast transcellular transport. Methods Mol Med. 2006;122:225–239. doi: 10.1385/1-59259-989-3:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowers GN, McComb RB. A continuous spectrophotometric method for measuring the serum activity of alkaline phophatase. Clin Chem. 1966;12:70–89. [PubMed] [Google Scholar]
- 5.Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caniggia I, Winter JL. Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies–a review. Placenta. 2002;23(Suppl A):S47–S57. doi: 10.1053/plac.2002.0815. [DOI] [PubMed] [Google Scholar]
- 7.Cassoni P, Sapino A, Munaron L, Deaglio S, Chini B, Graziani A, Ahmed A, Bussolati G. Activation of functional oxytocin receptors stimulates cell proliferation in human trophoblast and choriocarcinoma cell lines. Endocrinology. 2001;142:1130–1136. doi: 10.1210/endo.142.3.8047. [DOI] [PubMed] [Google Scholar]
- 8.Charnock-Jones DS, Sharkey AM, Bobcock CA, Ahmed A, Plevin R, Ferarra N, Smith SK. Vascular endothelial growth factor receptor localization and activation in human trophoblast and choriocarcinoma cells. Biol Reprod. 1994;51:524–530. doi: 10.1095/biolreprod51.3.524. [DOI] [PubMed] [Google Scholar]
- 9.Esterman A, Greco MA, Mitani Y, Finlay TH, Ismail-Beigi F, Dancis J. The effect of hypoxia on human trophoblast in culture: morphology, glucose transport and metabolism. Placenta. 1997;18:129–136. doi: 10.1016/s0143-4004(97)90084-9. [DOI] [PubMed] [Google Scholar]
- 10.Evseenko DA, Paxton JW, Keelan JA. ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1357–R1365. doi: 10.1152/ajpregu.00630.2005. [DOI] [PubMed] [Google Scholar]
- 11.Furesz TC, Smith CH, Moe AJ. ASC system activity is altered by development of cell polarity in trophoblast from human placenta. Am J Physiol Cell Physiol. 1993;265:C212–C217. doi: 10.1152/ajpcell.1993.265.1.C212. [DOI] [PubMed] [Google Scholar]
- 12.Gagnon R. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S99–S107. doi: 10.1016/s0301-2115(03)00179-9. [DOI] [PubMed] [Google Scholar]
- 13.Gaither K, Quraishi AN, Illsley NP. Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter. J Clin Endocrinol Metab. 1999;84:695–701. doi: 10.1210/jcem.84.2.5438. [DOI] [PubMed] [Google Scholar]
- 14.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 15.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 16.Hahn T, Barth S, Graf R, Engelman M, Beslagic D, Reul JM, Holsboer F, Dohr G, Desoye G. Placental glucose transporter expression is regulated by glucocorticoids. J Clin Endocrinol Metab. 1999;84:1445–1452. doi: 10.1210/jcem.84.4.5607. [DOI] [PubMed] [Google Scholar]
- 17.Hahn T, Barth S, Hofman W, Reich O, Lang I, Desoye G. Hyperglycemia regulates the glucose-transport system of clonal choriocarcinoma cells in vitro. A potential molecular mechanism contributing to the adjunct effect of glucose in tumor therapy. Int J Cancer. 1998;78:353–360. doi: 10.1002/(SICI)1097-0215(19981029)78:3<353::AID-IJC16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Hahn T, Barth S, Weiss U, Mosgoeller W, Desoye G. Sustained hyperglycemia in vitro down-regulates the GLUT1 glucose transport system of cultured human placental trophoblast: a mechanism to protect fetal development? FASEB J. 1998;12:1221–1231. doi: 10.1096/fasebj.12.12.1221. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi M, Sakata M, Takeda T, Yamamoto T, Okamoto Y, Sawada K, Kimura A, Minekawa R, Tahara M, Tasaka K, Murata Y. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1alpha under hypoxic conditions in trophoblast-derived cells. J Endocrinol. 2004;183:145–154. doi: 10.1677/joe.1.05599. [DOI] [PubMed] [Google Scholar]
- 20.Hickey MM, Simon MC. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Curr Top Dev Biol. 2006;76:217–257. doi: 10.1016/S0070-2153(06)76007-0. [DOI] [PubMed] [Google Scholar]
- 21.Hohn HP, Linke M, Ugele B, Denker HW. Differentiation markers and invasiveness: discordant regulation in normal trophoblast and choriocarcinoma cells. Exp Cell Res. 1998;244:249–258. doi: 10.1006/excr.1998.4184. [DOI] [PubMed] [Google Scholar]
- 22.Hohn HP, Parker CR, Jr, Boots LR, Denker HW, Hook M. Modulation of differentiation markers in human choriocarcinoma cells by extracellular matrix: on the role of a three-dimensional matrix structure. Differentiation. 1992;51:61–70. doi: 10.1111/j.1432-0436.1992.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 23.Illsley NP, Sellers MC, Wright RL. Glycaemic regulation of glucose transporter expression and activity in the human placenta. Placenta. 1998;19:517–524. doi: 10.1016/s0143-4004(98)91045-1. [DOI] [PubMed] [Google Scholar]
- 24.James JL, Stone PR, Chamley LW. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Hum Reprod Update. 2006;12:137–144. doi: 10.1093/humupd/dmi043. [DOI] [PubMed] [Google Scholar]
- 25.Janneau JL, Maldonado-Estrada J, Tachdjian G, Miran I, Motte N, Saulnier P, Sabourin JC, Cote JF, Simon B, Frydman R, Chaouat G, Bellet D. Transcriptional expression of genes involved in cell invasion and migration by normal and tumoral trophoblast cells. J Clin Endocrinol Metab. 2002;87:5336–5339. doi: 10.1210/jc.2002-021093. [DOI] [PubMed] [Google Scholar]
- 26.Jansson T, Cowley EA, Illsley NP. Cellular localization of glucose transporter messenger RNA in human placenta. Reprod Fertil Dev. 1995;7:1425–1430. doi: 10.1071/rd9951425. [DOI] [PubMed] [Google Scholar]
- 27.Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993;77:1554–1562. doi: 10.1210/jcem.77.6.8263141. [DOI] [PubMed] [Google Scholar]
- 28.Jansson T, Ylven K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23:392–399. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- 29.Jones HN, Ashworth CJ, Page KR, McArdle HJ. Cortisol stimulates system A amino acid transport and SNAT2 expression in a human placental cell line (BeWo) Am J Physiol Endocrinol Metab. 2006;291:E596–E603. doi: 10.1152/ajpendo.00359.2005. [DOI] [PubMed] [Google Scholar]
- 30.Joost HG, Bell G, Best J, Birnbaum M, Charron M, Chen Y, Doege H, James D, Lodish H, Moley K, Moley J, Mueckler M, Rogers S, Schürmann A, Seino S, Thorens B. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab. 2002;282:E974–E976. doi: 10.1152/ajpendo.00407.2001. [DOI] [PubMed] [Google Scholar]
- 31.Kingdom JC, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18:613–623. doi: 10.1016/s0143-4004(97)90000-x. [DOI] [PubMed] [Google Scholar]
- 32.Kudo Y, Boyd CAR. Changes in expression and function of syncytin and its receptor, amino acid transport system B(0) (ASCT2), in human placental choriocarcinoma BeWo cells during syncytialization. Placenta. 2002;23:536–541. doi: 10.1053/plac.2002.0839. [DOI] [PubMed] [Google Scholar]
- 33.Kudo Y, Boyd CAR, Sargent IL, Redman CWG. Hypoxia alters expression and function of syncytin and its receptor during trophoblast cell fusion of human placental BeWo cells: implications for impaired trophoblast syncytialisation in preeclampsia. Biochim Biophys Acta. 2003;1638:63–71. doi: 10.1016/s0925-4439(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 34.Kunst A, Draeger B, Ziegenhorn J. Glucose: UV-methods with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer H, editor. Methods in Enzymatic Analysis. Deerfield Beach, FL: Verlag Chemie; 1984. pp. 163–172. [Google Scholar]
- 35.Leitner K, Ellinger I, Grill M, Brabec M, Fuchs R. Efficient apical IgG recycling and apical-to-basolateral transcytosis in polarized BeWo cells overexpressing hFcRn. Placenta. 2006;27:799–811. doi: 10.1016/j.placenta.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Gu Y, Zhang Y, Lucas MJ, Wang Y. High glucose levels down-regulate glucose transporter expression that correlates with increased oxidative stress in placental trophoblast cells in vitro. J Soc Gynecol Investig. 2004;11:75–81. doi: 10.1016/j.jsgi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Liu F, Soares MJ, Audus KL. Permeability properties of monolayers of the human trophoblast cell line, BeWo. Am J Physiol Cell Physiol. 1997;273:C1596–C1604. doi: 10.1152/ajpcell.1997.273.5.C1596. [DOI] [PubMed] [Google Scholar]
- 38.Mandl M, Ghaffari-Tabrizi N, Haas J, Nohammer G, Desoye G. Differential glucocorticoid effects on proliferation and invasion of human trophoblast cell lines. Reproduction. 2006;132:159–167. doi: 10.1530/rep.1.00976. [DOI] [PubMed] [Google Scholar]
- 39.Matouskova M, Blazkova J, Pajer P, Pavlicek A, Hejnar J. CpG methylation suppresses transcriptional activity of human syncytin-1 in non-placental tissues. Exp Cell Res. 2006;312:1011–1020. doi: 10.1016/j.yexcr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Moe AJ, Furesz TC, Smith CH. Functional characterization of L-alanine transport in a placental choriocarcinoma cell line (BeWo) Placenta. 1994;15:797–802. doi: 10.1016/s0143-4004(05)80182-1. [DOI] [PubMed] [Google Scholar]
- 41.Myatt L. Role of placenta in preeclampsia. Endocrine. 2002;19:103–111. doi: 10.1385/ENDO:19:1:103. [DOI] [PubMed] [Google Scholar]
- 42.Novak D, Quiggle F, Haafiz A. Impact of forskolin and amino acid depletion upon System A activity and SNAT expression in BeWo cells. Biochimie. 2006;88:39–44. doi: 10.1016/j.biochi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Ogura K, Sakata M, Okamoto Y, Yasui Y, Tadokoro C, Yoshimoto Y, Yamaguchi M, Kurachi H, Maeda T, Murata Y. 8-Bromo-cyclicAMP stimulates glucose transporter-1 expression in a human choriocarcinoma cell line. J Endocrinol. 2000;164:171–178. doi: 10.1677/joe.0.1640171. [DOI] [PubMed] [Google Scholar]
- 44.Palmer ME, Watson AL, Burton GJ. Morphological analysis of degeneration and regeneration of syncytiotrophoblast in first trimester placental villi during organ culture. Hum Reprod. 1997;12:379–382. doi: 10.1093/humrep/12.2.379. [DOI] [PubMed] [Google Scholar]
- 45.Peebles DM. Fetal consequences of chronic substrate deprivation. Semin Fetal Neonatal Med. 2004;9:379–386. doi: 10.1016/j.siny.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Sakata M, Kurachi H, Imai T, Tadokoro C, Yamaguchi M, Yoshimoto Y, Oka Y, Miyake A. Increase in human placental glucose transporter-1 during pregnancy. Eur J Endocrinol. 1995;132:206–212. doi: 10.1530/eje.0.1320206. [DOI] [PubMed] [Google Scholar]
- 47.Semenza GL. Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol. 2006;91:803–806. doi: 10.1113/expphysiol.2006.033498. [DOI] [PubMed] [Google Scholar]
- 48.Simán CM, Sibley CP, Jones CJP, Turner MA, Greenwood SL. The functiona regeneration of syncytiotrophoblast in cultured explants of term placenta. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1116–R1122. doi: 10.1152/ajpregu.2001.280.4.R1116. [DOI] [PubMed] [Google Scholar]
- 49.Sooranna SR, Oteng-Ntim E, Meah R, Ryder TA, Bajoria R. Characterization of human placental explants: morphological, biochemical and physiological studies using first and third trimester placenta. Hum Reprod. 1999;14:536–541. doi: 10.1093/humrep/14.2.536. [DOI] [PubMed] [Google Scholar]
- 50.Tobin KAR, Harsem NK, Dalen KT, Staff AC, Nebb HI, Duttaroy AK. Regulation of ADRP expression by long-chain polyunsaturated fatty acids in BeWo cells, a human placental choriocarcinoma cell line. J Lipid Res. 2006;47:815–823. doi: 10.1194/jlr.M500527-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem. 2002;277:27975–27981. doi: 10.1074/jbc.M204152200. [DOI] [PubMed] [Google Scholar]
- 52.Triantafyllou A, Liakos P, Tsakalof A, Georgatsou E, Simos G, Bonanou S. Cobalt induces hypoxia-inducible factor-1alpha (HIF-1alpha) in HeLa cells by an iron-independent, but ROS-, PI-3K- and MAPK-dependent mechanism. Free Radic Res. 2006;40:847–856. doi: 10.1080/10715760600730810. [DOI] [PubMed] [Google Scholar]
- 53.Vardhana P, Illsley N. Transepithelial glucose transport and metabolism in BeWo choriocarcinoma cells. Placenta. 2002;23:653–660. doi: 10.1053/plac.2002.0857. [DOI] [PubMed] [Google Scholar]
- 54.Wice B, Menton D, Geuze H, Schwartz AL. Modulators of cyclic AMP metabolism induce syncytiotrophoblast formation in vitro. Exp Cell Res. 186:306–316. doi: 10.1016/0014-4827(90)90310-7. [DOI] [PubMed] [Google Scholar]
- 55.Yu C, Shen K, Lin M, Chen P, Lin C, Chang GD, Chen H. GCMa regulates the syncytin-mediated trophoblastic fusion. J Biol Chem. 2002;277:50062–50068. doi: 10.1074/jbc.M209316200. [DOI] [PubMed] [Google Scholar]
- 56.Zamudio S, Baumann MU, Illsley NP. Effects of chronic hypoxia in vivo on the expression of human placental glucose transporters. Placenta. 2006;27:49–55. doi: 10.1016/j.placenta.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zelzer E, Levy Y, Kahana C, Shilo B, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]