Table 1.
FLP-mediated hydrogenation of imines. 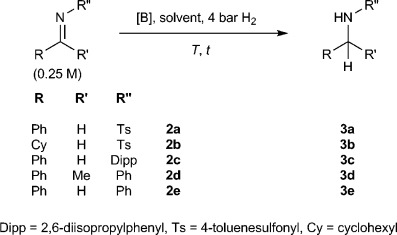
| Entry | Substrate | Solvent | T [°C] | [B] (mol %) | t [h] | Yield [%][a] |
|---|---|---|---|---|---|---|
| 1[bc] | 2 a | C7H8 | 80 | 1 a (10) | 22 | 7 |
| 2[bd] | 2 a | C7H8 | 80 | 1 a (10) | 22 | 99 |
| 3 | 2 a | [D8]THF | 60 | 1 b (5) | 3 | >99 (98)[e] |
| 4 | 2 b | [D8]THF | 60 | 1 b (5) | 3 | >99 |
| 5 | 2 a | THF | 60 | 1 b (5) | 3 | >99[f] |
| 6 | 2 c | [D8]THF | 60 | 1 b (5) | 8 | >99 (99)[e] |
| 7 | 2 d | [D8]THF | 80 | 1 b (5) | 18 | 71 |
| 8 | 2 e | [D8]THF | 60 | 1 b (15) | 8 | 91 |
| 9 | 2 a | C7D8 | 60 | 1 b (5) | 3 | 0 |
| 10 | 2 b | C7D8 | 60 | 1 b (5) | 3 | 0 |
| 11 | 2 c | C7D8 | 60 | 1 b (5) | 8 | 0 |
| 12 | 2 d | C7D8 | 80 | 1 b (5) | 18 | 79 |
| 13 | 2 e | C7D8 | 60 | 1 b (15) | 8 | 26 |
| 14 | 2 a | Dioxane | 60 | 1 b (5) | 41 | 96 |
| 15 | 2 a | [D8]THF | 60 | 1 c (5) | 72 | 90 |
| 16 | 2 a | [D8]THF | 80 | 1 a (10) | 72 | 84 |
| 17 | 2 a | [D8]THF | 80 | 1 d (5) | 72 | 0 |
Yields measured by in situ 1H NMR spectroscopy, using 1,3,5-trimethoxybenzene in C6D6 in a capillary insert as an internal integration standard.
Result reported by Klankermayer and Chen.[25a]
10 bar H2.
30 bar H2.
Number in parentheses is yield isolated after increasing to 1 mmol scale (see Supporting Information).
Initial reaction mixture prepared using pre-dried solvent under air (see Supporting Information).
