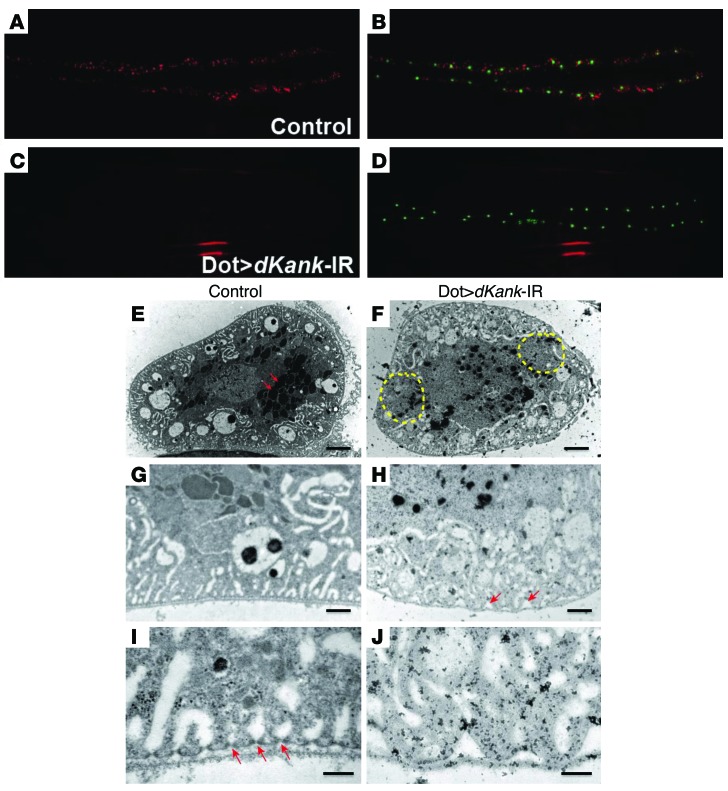
Figure 3. dKank is required for Drosophila cardiac nephrocyte function and cytoskeleton structure.
(A and B) Control Drosophila larvae carrying myosin heavy chain promoter–driven ANF-RFP (red), which is secreted from muscles into hemolymph and then filtered and taken up by cardiac nephrocytes. Hand-GFP labels the nuclei of nephrocytes. (C) Nephrocytes with dKank RNAi knockdown using Dot-Gal4 (Dot>dKank-IR) show substantially reduced uptake of ANF-RFP, (D) whereas the number and size of nephrocytes remain the same as those of wild-type nephrocytes. (E) Control third instar Drosophila larvae nephrocytes show evenly distributed vesicles (arrows) and vacuoles in distinct layers of cytoplasm. (F) Nephrocytes with dKank RNAi exhibit uneven distribution of endocytic vacuoles and lacuna channels, with reduced numbers of vesicles and vacuoles in some areas (yellow circles) but increased vesicles and vacuoles in other areas as well as reduced numbers of lysosomes. (G) High-magnification image of control nephrocytes showing even and flat plasma membrane and adjacent basement membrane. (H) Nephrocytes with dKank RNAi show wave-shaped plasma membrane surface and gaps between the plasma membrane and the basement membrane (arrows). Distribution of lacuna channels close to the nephrocyte cell surface is disrupted. (I) High-magnification images of control nephrocytes showing evenly distributed electron-dense slit diaphragm (arrows) and lacuna channel structures. (J) Nephrocytes with dKank RNAi show wave-shaped plasma membrane surface, without slit diaphragm structures and abnormally shaped lacuna channels. Original magnification, ×20 (A–D). Scale bar: 4 μm (E and F); 1 μm (G and H); 0.2 μm (I and J).