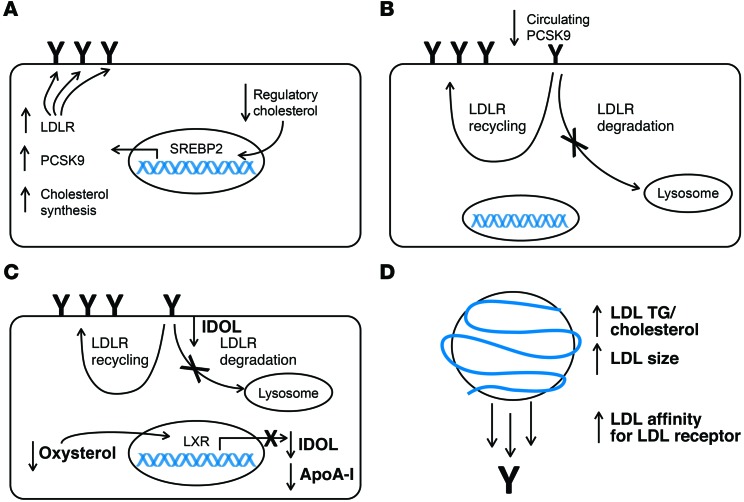
Figure 4. Potential mechanisms responsible for the increase in the LDL ApoB FCR observed in response to anacetrapib treatment.
(A) A reduction in the cholesterol content of the regulatory pool of intracellular cholesterol activates SREBP2, leading to increased transcription of LDLR, PCSK9, and cholesterol synthetic genes. Increased LDLR transcription increases the amount of LDLR at the cell surface of hepatocytes (indicated by “Y”), leading to an increase in LDL clearance. This scenario should be accompanied by an increase in the PCSK9 PR and cholesterol synthesis as reflected by lathosterol levels. (B) Reduced levels of PCSK9 circulating in plasma results in less targeting of the LDLR for degradation and an increase in LDL recycling. This would increase the amount of LDLR at the cell surface of hepatocytes and lead to an increase in LDL clearance. (C) A decrease in hepatic oxysterols reduces activation of the LXR, leading to reduced transcription of MYLIP, the gene that encodes IDOL, and APOA1. Reduced IDOL at the plasma membrane attenuates LDLR degradation and increases LDL recycling, leading to an increased number of LDLRs at the hepatocyte cell surface and, consequently, an increase in LDL clearance. This scenario should be accompanied by a reduced ApoA-I PR. (D) An increase in the TG/cholesterol ratio as well as an increase in LDL particle size as seen by NMR increases the affinity of LDL for the LDLR, leading to a greater degree of LDL binding to the LDLR and an increase in overall LDL clearance.