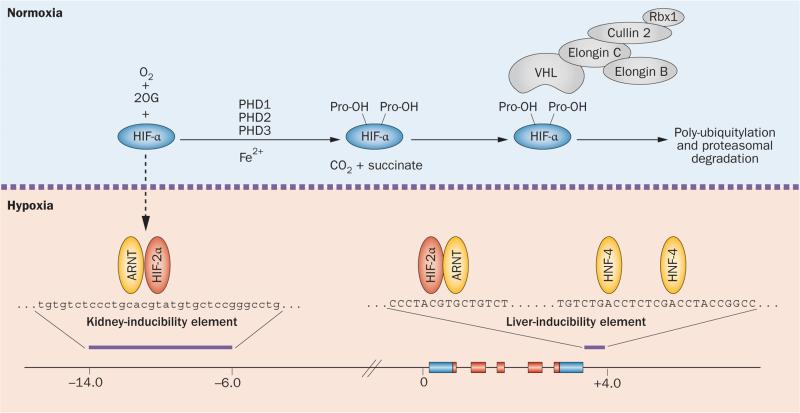
Figure 2.
Regulation of HIF degradation by PHDs and hypoxic induction of erythropoietin. HIF controls a wide spectrum of tissue-specific and systemic hypoxia responses, with HIF-2 being the principal regulator of EPO transcription in vivo. The HIF oxygen-sensing machinery targets HIF-1α, HIF-2α and HIF-3α for proteasomal degradation by the VHL–E3-ubiquitin ligase complex. In the presence of oxygen HIF-α is hydroxylated at specific proline residues (Pro-OH) by PHD enzymes. Hydroxylation of HIF-α permits binding to the β-domain of VHL, which functions as the substrate recognition component of the VHL–E3-ubiquitin ligase complex. Under hypoxic conditions, HIF-α degradation is inhibited and HIF-α translocates to the nucleus, where it forms a heterodimer with ARNT and binds hypoxia regulatory elements in EPO, which contain the HIF consensus binding site 5'-RCGTG-3'. The hypoxic induction of EPO in the liver is mediated by the liver-inducibility element in the 3'-end of the EPO gene, whereas the hypoxic induction of renal EPO requires the kidney inducibility element located upstream of the EPO transcription start site. The putative DNA sequence of the kidney inducibility element is shown in lower case letters. HNF-4 is an important coregulator of EPO. Boxes depict EPO exons. EPO coding sequences and non-translated sequences are depicted in red and blue, respectively. The distance from the EPO transcription start site is indicated in kilobases. Abbreviations: 2OG, 2-oxoglutarate; ARNT, aryl hydrocarbon receptor nuclear translocator; EPO, erythropoietin gene; Fe2+, ferrous iron; HIF, hypoxia-inducible factor; HNF-4, hepatocyte nuclear factor 4; PHD, prolyl-4-hydroxylase domain.