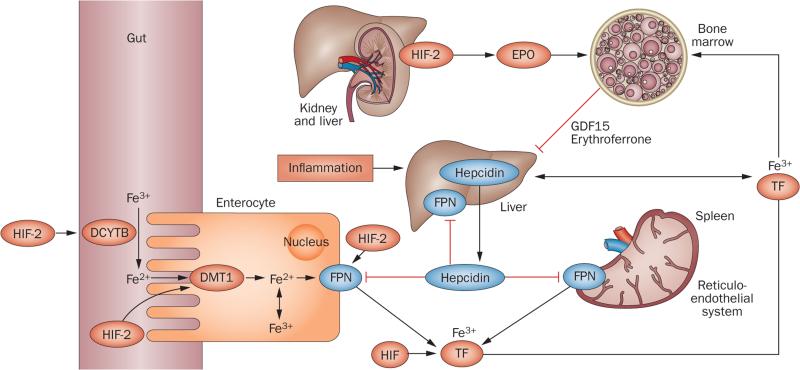
Figure 3.
HIF coordinates erythropoietin production with iron metabolism. HIF-2 stimulates renal and hepatic erythropoietin synthesis, which raises serum erythropoietin levels, stimulating erythropoiesis in the bone marrow. In the duodenum, DCYTB reduces Fe3+ to Fe2+, which then enters enterocytes via DMT1. DCYTB and DMT1 are both regulated by HIF-2. Iron is then released into the circulation via FPN, which is also HIF-2-inducible. In the circulation iron is transported in a complex with TF to the liver, bone marrow and other organs; cells of the reticuloendothelial system acquire iron through the phagocytosis of senescent red cells. TF is HIF-regulated, and hypoxia and/or pharmacologic PHD inhibition raises TF serum levels. Increased erythropoietic activity in the bone marrow produces GDF15 and erythroferrone, which suppress hepcidin in hepatocytes. Hepcidin suppression increases FPN expression on enterocytes, hepatocytes and macrophages, resulting in increased iron absorption and mobilization from internal stores. Inflammation stimulates hepcidin production in the liver and leads to reduced FPN expression and hypoferraemia. In addition to regulating hepcidin indirectly by stimulating erythropoiesis, in vitro studies suggest that HIF might also modulate hepcidin expression through the regulation of furin and transmembrane protease serine 6.240–244 Abbreviations: DCYTB, duodenal cytochrome b reductase 1; DMT1, divalent metal transporter-1; EPO, plasma erythropoietin; Fe2+, ferrous iron; Fe3+, ferric iron; FPN, ferroportin; GDF15, growth differentiation factor 15; HIF, hypoxia-inducible factor; TF, transferrin.