Background: Caudal is a core promoter-preferential transcription factor.
Results: We defined functional regions in Caudal that are important for core promoter-preferential activation and demonstrated that dCBP confers core promoter-preferential activation.
Conclusion: The unique combination of Caudal and dCBP enables co-activation in a core promoter-preferential manner.
Significance: We provide mechanistic insights into core promoter-enhancer compatibility.
Keywords: developmental factor, Drosophila, gene expression, RNA polymerase II, transcription regulation, CBP, Caudal, DPE, core promoter, fushi tarazu
Abstract
Regulation of RNA polymerase II transcription is critical for the proper development, differentiation, and growth of an organism. The RNA polymerase II core promoter is the ultimate target of a multitude of transcription factors that control transcription initiation. Core promoters encompass the RNA start site and consist of functional elements such as the TATA box, initiator, and downstream core promoter element (DPE), which confer specific properties to the core promoter. We have previously discovered that Drosophila Caudal, which is a master regulator of genes involved in development and differentiation, is a DPE-specific transcriptional activator. Here, we show that the mouse Caudal-related homeobox (Cdx) proteins (mCdx1, mCdx2, and mCdx4) are also preferential core promoter transcriptional activators. To elucidate the mechanism that enables Caudal to preferentially activate DPE transcription, we performed structure-function analysis. Using a systematic series of deletion mutants (all containing the intact DNA-binding homeodomain) we discovered that the C-terminal region of Caudal contributes to the preferential activation of the fushi tarazu (ftz) Caudal target gene. Furthermore, the region containing both the homeodomain and the C terminus of Caudal was sufficient to confer core promoter-preferential activation to the heterologous GAL4 DNA-binding domain. Importantly, we discovered that Drosophila CREB-binding protein (dCBP) is a co-activator for Caudal-regulated activation of ftz. Strikingly, dCBP conferred the ability to preferentially activate the DPE-dependent ftz reporter to mini-Caudal proteins that were unable to preferentially activate ftz transcription themselves. Taken together, it is the unique combination of dCBP and Caudal that enables the co-activation of ftz in a core promoter-preferential manner.
Introduction
Embryonic development is highly dependent on transcriptional regulation (for reviews, see Refs. 1–5). The accurate expression of developmental control genes involves nucleosome remodeling, histone modifications, and the binding of transcriptional activators and co-activators to enhancers and promoters (for reviews, see Refs. 2, 6–9). The precise recruitment of RNA polymerase II (Pol II)3 to the transcription start site (TSS) plays a central role in regulating gene expression (10–18). Transcription initiation in eukaryotes requires the assembly of basal transcription factors and RNA polymerase II at the core promoter region to form the preinitiation complex (for reviews, see Refs. 19–21). The core promoter is defined as the region from −40 to +40 relative to the TSS that is required for accurate initiation of transcription by RNA polymerase II (10–18). Core promoters are highly diverse in structure and function. The core promoter may contain one or more short DNA sequences, termed core promoter elements or motifs, such as the TATA box (22), initiator (Inr) (23), TCT (24), motif 10 element (25, 26), and downstream core promoter element (DPE) (27–29), which contribute to its function. There are no universal core promoter elements. The TATA box, the first eukaryotic core promoter element identified (22), is conserved from Archaea to humans (30). The upstream T is typically located at −31 or −30 relative to the TSS (31, 32). It is bound by the TATA-binding protein subunit of the TFIID complex, which is the first basal transcription factor that binds the promoter in the hierarchical recruitment of RNA Pol II (21, 33). The Inr encompasses the TSS and is considered the most common core promoter element (34–36). The Inr serves as recognition site for the TAF1 and TAF2 subunits of TFIID (37). The DPE was originally identified as a TFIID recognition site that is downstream of the Inr (precisely located at +28 to +33 relative to the A+1 of the Inr) and is conserved from Drosophila to humans (27–29). DPE-dependent transcription is highly dependent on the Inr element (27–29).
The majority of the Drosophila Hox genes, which lack TATA box elements, contain functional DPE motifs (38). Moreover, Drosophila Caudal, a sequence-specific homeodomain transcription factor and key regulator of the Hox genes, has been demonstrated to activate its target promoters with a preference for a DPE motif (38). The Drosophila caudal gene encodes a homeodomain transcription factor expressed in a gradient-like manner at the posterior of the embryo (39–43). Mutations in caudal that reduce or eliminate the gradient cause abnormal zygotic expression of several segmentation genes and alter the global body pattern (41). In addition, ectopic expression of caudal in Drosophila disrupts head development and segmentation (44) probably due to the fact that Caudal is a direct activator of the pair rule segmentation gene fushi tarazu (ftz) (45). The Caudal protein activates ftz transcription in the posterior half of the embryo by interacting with multiple copies of TTTATG consensus sequence located upstream of the TSS (45).
caudal-like genes are highly conserved in evolution and have been found in multiple species (41, 46–57). The vertebrate Caudal-related homeobox (Cdx) proteins have been identified as factors that mediate anterior-posterior patterning through Hox gene regulation (46, 50, 52, 53, 55, 56). Moreover, recent studies suggest that Cdx family members are involved in the proliferation and differentiation of hematopoietic cells (58–61).
Here, we show that the ability to activate ftz transcription with a preference for a DPE motif is conserved between Drosophila Caudal and mouse Caudal-related Cdx proteins. The mouse Cdx (mCdx) proteins are the first identified vertebrate transcription factors that have the ability to preferentially activate DPE-dependent promoters. To understand the mechanism of core promoter-preferential activation by Caudal, we performed a structure-function analysis. Our results suggest that the C terminus of Caudal and the N termini of mCdx1 and mCdx2 are important for core promoter-preferential activation. Moreover, we discovered that Drosophila cAMP-response element-binding protein (CREB)-binding protein (dCBP) is a co-activator for Caudal-regulated activation of ftz and have mapped the region necessary for the co-activation to the N terminus of dCBP. Taken together, our analysis indicates that dCBP is involved in Caudal-mediated enhancer-promoter specificity and provides mechanistic insights into core promoter-preferential activation by Caudal.
Experimental Procedures
Plasmid Construction
GAL4-VP16 and GAL4-stop were each subcloned into the pAc5.1 expression vector by PCR using the GAL4-VP16 pJL2 plasmid as a template.
GAL4 DNA-binding domain (DBD)-full-length Caudal (amino acids (aa) 2–427), GAL4-DBD-Caudal N-term. + homeodomain (HD) (aa 2–334), GAL4-DBD-Caudal HD + C-term. (aa 270–427), GAL4-DBD-Caudal C-term. (aa 363–427), and GAL4-DBD-Caudal HD (aa 270–334) were each subcloned into the pAc5.1 expression vector by PCR using the pAcFLAG-full-length Caudal plasmid as a template. Drosophila wild-type CBP and a previously characterized HAT domain mutant (F2161A point mutation) (62) (kindly provided by Dr. Sarah Smolik, Oregon Health and Science University) were subcloned into the pAc5.1 expression vector using restriction enzymes. Drosophila CBP Δ2–600, dCBP Δ2–1020, and dCBP Δ1382–1595 were generated by site-directed mutagenesis using the Stratagene QuikChange protocol and each expressed using the pAc5.1 expression vector. Full-length Bicoid was subcloned into the pAc5.1 expression vector with C-terminal V5 and His6 tags for expression in Drosophila S2R+ cells. All plasmid sequences were verified by sequencing.
GAL4-responsive firefly luciferase reporter plasmids were constructed by cloning five GAL4 DNA-binding sites upstream of the minimal ftz promoter (from −40 to +40 relative to the TSS) that drives the expression of the firefly luciferase reporter gene. The ftz minimal promoter is either DPE-dependent (containing a mutated TATA box (mTATA)) or TATA-dependent (containing a mutated DPE (mDPE)). The distance between the downstream-most GAL4 DNA-binding site and the −40 position of the ftz minimal promoter is 21 bp.
Construction of Mouse Cdx1 and Cdx2 Expression Vectors
Mouse Cdx1 and Cdx2 were kindly provided by Dr. David Lohnes (University of Ottawa). The complementary DNAs (cDNAs) of mCdx1 and mCdx2 were each subcloned into the pAc5.1 expression vector for expression in Drosophila S2R+ cells using restriction enzymes. GAL4-DBD-full-length Cdx1 (aa 2–268), GAL4-DBD-Cdx1 N-term. + HD (aa 2–215), and GAL4-DBD-Cdx1 HD + C-term. (aa 151–268) were each subcloned into the pAc5.1 expression vector by PCR using the pAcCdx1 plasmid as a template. GAL4-DBD-full-length Cdx2 (aa 2–311), GAL4-DBD-Cdx2 N-term. + HD (aa 2–246), and GAL4-DBD-Cdx2 HD + C-term. (aa 182–311) were each subcloned into the pAc5.1 expression vector by PCR using the pAcCdx2 plasmid as a template.
RNA Isolation and Nested PCR toward the Cloning of Mouse cdx4
Mouse embryo (E7.5) was used for RNA extraction using the TRIzol reagent (Life Technologies). cDNA was synthesized using oligo(dT) primers. Because mCdx4 PCR amplification was problematic, a nested PCR approach was used with the following primers: outer primers: forward, 5′-CTCAGGATGGCTTAAGGGGC-3′; reverse, 5′-GCCCCCATATGACAGCATGG-3′; inner primers: forward, 5′-GCGGAATTCATGTATGGAAGCTGCCTTTTAG-3′; reverse, 5′-TCGCGGCCGCTCATTCAGAAACTATGACCTGCTG-3′. The cDNA of mCdx4 was subcloned into the pAc5.1 expression vector for expression in Drosophila S2R+ cells using restriction enzymes. GAL4-DBD-full-length Cdx4 (aa 2–282), GAL4-DBD-Cdx4 N-term. + HD (aa 2–232), and GAL4-DBD-Cdx4 HD + C-term. (aa 168–282) were each subcloned into the pAc5.1 expression vector by PCR using the pAcCdx4 plasmid as a template.
Transfections and Reporter Gene Assays
Drosophila Schneider S2R+ adherent cells were cultured in Schneider's Drosophila medium (Biological Industries) that was supplemented with 10% heat-inactivated FBS. Cells were transfected in 24-well plates by using the Escort IV reagent (Sigma). For Dual-Luciferase assays, cells were plated at 0.6 × 106 cells/well of a 24-well plate 1 day prior to transfection. Each well was transfected with a total of 930 ng of a single expression vector or a vector control as indicated, 60 ng of firefly luciferase reporter constructs, and 10 ng of Pol III-Renilla luciferase reporter (kindly provided by Dr. Norbert Perrimon, Harvard Medical School). Co-activation experiments were performed by transfection of 465 ng of each expression vector (Caudal, dCBP, or vector control) using a total of 930 ng of expression vectors. Medium was replaced the next morning, and cells were harvested 36–48 h post-transfection and assayed for Dual-Luciferase activities as specified by the manufacturer (Promega). To correct for variations in transfection efficiency, the firefly luciferase activity of each sample was normalized to the corresponding Renilla luciferase activity. Each transfection was performed in triplicate. Each graph represents an average of at least three independent experiments (as indicated in each figure legend).
Statistical Analyses
Statistical analyses were performed using SPSS. The analyses of the differences between the activity of an mDPE reporter and an mTATA reporter for a specific activator were performed using Student's t test. The comparison between different activators was done using two-way analysis of variance.
Results
The Caudal Vertebrate Homologues Mouse Cdx1, Cdx2, and Cdx4 Are Core Promoter-preferential Activators
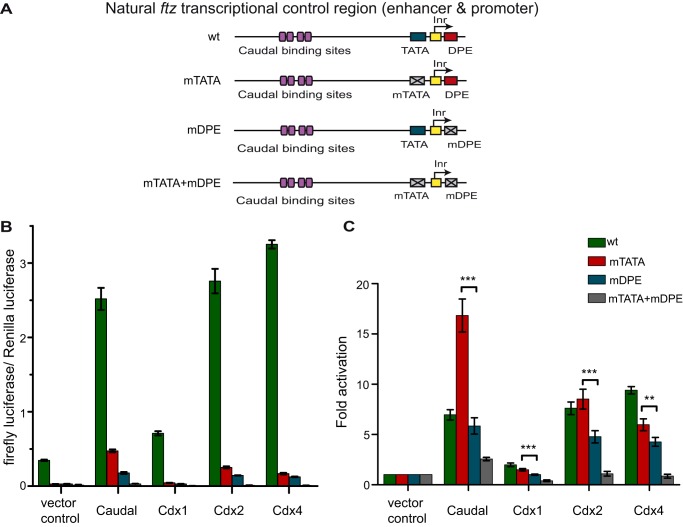
Drosophila Caudal is the first core promoter-specific activator discovered that preferentially activates DPE-specific transcription (38). To gain further insight into Caudal function, we decided to check whether the core promoter-preferential activation is conserved in the vertebrate homologs of Drosophila Caudal, the Cdx family members. There are three Caudal-related Cdx family members, Cdx1, Cdx2, and Cdx4, that possess both unique and common properties (46, 50, 52, 53, 55, 56, 58–61, 63–69). We compared the ability of the Cdx family members to preferentially activate transcription from the ftz enhancer and promoter, which naturally contains functional Inr, TATA box, and DPE core promoter elements and has been shown to be regulated by Drosophila Caudal in a core promoter-preferential manner (38). To that end, we used firefly luciferase reporter genes driven by either wild-type (WT), a DPE-dependent (containing mTATA), a TATA-dependent (containing mDPE), or a double mutant (mTATA + mDPE) version of the ftz transcriptional control region from −988 to +40 relative to the RNA start site (38), which includes the previously characterized Caudal-binding sites (45). As Drosophila melanogaster Schneider cells (S2R+) do not express endogenous caudal (FlyBase), they are ideally suited for this study. Drosophila S2R+ cells were co-transfected with firefly luciferase reporters driven by a ftz genomic fragment containing either WT, mTATA, mDPE, or mTATA + mDPE (depicted in Fig. 1A) as well as with a Pol III-Renilla luciferase reporter vector (to normalize for variations in transfection efficiency) and vectors driving the expression of either Drosophila Caudal, mCdx1, mCdx2, or mCdx4. Cell extracts were assayed for Dual-Luciferase activity. The normalized firefly to Renilla luciferase activities are presented in Fig. 1B, and the -fold activation by Caudal, Cdx1, Cdx2, and Cdx4 relative to the activities of the promoters in the absence of a co-transfected Caudal or Cdx expression plasmid (which were defined to be 1) are presented in Fig. 1C.
FIGURE 1.
The Caudal vertebrate homologues mouse Cdx1, Cdx2, and Cdx4 are core promoter-preferential activators. Drosophila S2R+ cells were co-transfected with expression vectors for either full-length Drosophila Caudal or mouse Cdx1, Cdx2, or Cdx4 expression vectors as well as firefly luciferase reporter constructs driven by the ftz enhancer-promoter containing either the WT sequence, mTATA, mDPE, or mTATA + mDPE. A, schematic representation of the natural ftz transcriptional control region. The ftz core promoter contains both DPE and TATA box motifs. The reporter constructs contain ftz enhancer and promoter sequences from −988 to +40 relative to the +1 start site and are identical except for having a WT sequence, mTATA, mDPE, or mTATA + mDPE as depicted. To normalize for variations in transfection efficiency, cells were co-transfected with a Pol III-Renilla luciferase control plasmid and assayed for Dual-Luciferase activity. B, normalized firefly to Renilla luciferase activities. C, the luciferase activities depicted in B are reported relative to the activities of the promoters in the absence of a co-transfected Caudal or Cdx expression plasmid, which were defined to be 1. The graph represents an average of three independent experiments. Error bars represent S.E. **, 0.001 ≤ p ≤ 0.01; ***, p ≤ 0.001.
Consistent with previous observations (38), the wild-type ftz promoter exhibited a much higher basal transcriptional activity in the absence of transfected Caudal or Cdx expression vectors as compared with the mutant versions of the ftz promoter, indicating the dependence of the ftz promoter activity on both the TATA box and the DPE (Fig. 1B). Notably, mutation of both the TATA box and the DPE nearly abolished transcriptional activity. Remarkably, similarly to Drosophila Caudal, mCdx2 preferentially activated the DPE-dependent ftz reporter (Fig. 1C). It is of note that although the absolute levels of transcriptional activation of the WT reporter by Caudal were higher than the activation by Caudal of the mutated reporters (Fig. 1B) the -fold activation of the WT reporter by Caudal relative to the vector control was lower than that of the mTATA reporter (Fig. 1C). This likely results from the fact that the basal activity of the WT reporter is much higher than the basal activities of the mutant reporters, and the activation of the WT reporter by Caudal might have reached a maximum due to limiting factors in the cells.
Interestingly, co-transfection of mCdx1 preferentially activated the transcription of the DPE-dependent ftz reporter as compared with the TATA-dependent ftz reporter, albeit to overall lower levels than activation by either Drosophila Caudal or mCdx2 (Fig. 1C). Notably, mCdx4, which differs from mCdx1 and mCdx2 (67, 70, 71), activated ftz transcription with core promoter specificity; however, the -fold difference between the activation of the two reporters by mCdx4 was not as pronounced as that observed with mCdx1 or mCdx2 (Fig. 1C). Hence, core promoter-preferential activation of transcription is a conserved characteristic of Caudal and its related family members, mouse Cdx1, Cdx2, and Cdx4, which are shown here to be DPE-specific preferential activators.
The C-terminal Region of Caudal Contributes to Core Promoter-preferential Activation of ftz
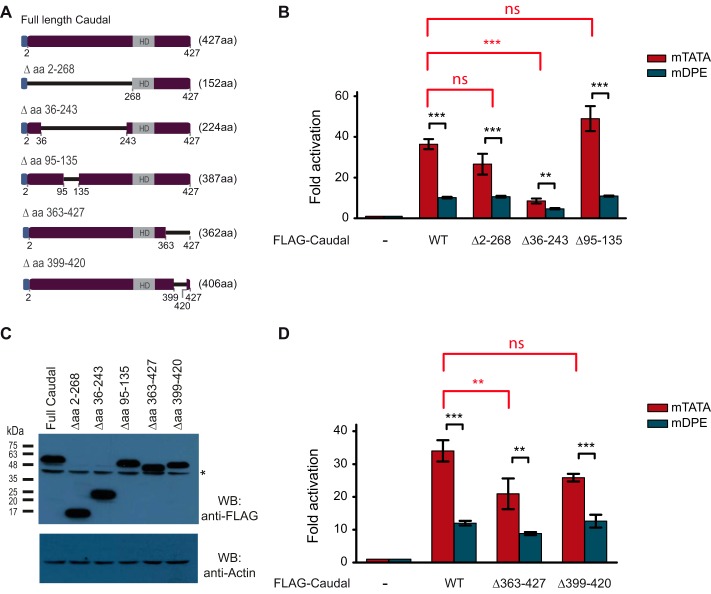
Caudal is an HD transcription factor. Using multiple motif/domain search bioinformatics tools, we did not find additional putative domains in the primary sequence of Caudal. To identify domains within the Drosophila Caudal protein that are important for preferential activation of DPE transcription, we constructed a series of FLAG-tagged Caudal deletion mutants in which multiple regions of the N-terminal and C-terminal ends were deleted but the DNA-binding HD was preserved (Fig. 2A). The regions we chose to delete had varying degrees of sequence conservation as compared with the vertebrate Cdx proteins. The expression vector for each deletion mutant was co-transfected into S2R+ cells with either ftz mTATA or ftz mDPE firefly luciferase reporter vectors as described previously.
FIGURE 2.
The C-terminal region of Caudal is important for core promoter-preferential activation of ftz. Drosophila S2R+ cells were co-transfected with expression vectors for either full-length Drosophila Caudal or Caudal deletion constructs as well as with firefly luciferase reporter constructs driven by the ftz enhancer-promoter containing either an mTATA or mDPE motif. To normalize for variations in transfection efficiency, cells were co-transfected with a Pol III-Renilla luciferase control plasmid and assayed for Dual-Luciferase activity. The activities are reported relative to the promoters in the absence of a co-transfected Caudal expression plasmid, which were defined to be 1. A, schematic representation of the full-length FLAG-Caudal protein and the Caudal mutants harboring N-terminal or C-terminal deletions. The blue box at the N terminus of the Caudal expression constructs denotes a FLAG tag. B, transcriptional activation of the ftz reporter gene by the full-length FLAG-Caudal and N-terminal deletion constructs (n = 3). C, Western blot (WB) analysis of Caudal expression vectors in S2R+ cells. Equal amounts of total protein were subjected to Western blot analysis with anti-FLAG antibodies. Actin served as a loading control. The asterisk denotes a cross-reactive band. D, transcriptional activation of the ftz reporter gene by the full-length FLAG-Caudal and C-terminal deletion constructs (n = 3). In all panels, error bars represent S.E. ns, p > 0.05; **, 0.001 ≤ p ≤ 0.01; ***, p ≤ 0.001.
Deletion of aa 2–268 did not significantly reduce the activation of the ftz mTATA or ftz mDPE firefly luciferase reporters as compared with the full-length Caudal (Fig. 2B). Surprisingly, an internal deletion of aa 36–243 (which are included in the N-terminal deletion of 2–268) led to a significant reduction in the ftz mTATA reporter activity as compared with that of full-length Caudal. Western blot analysis using anti-FLAG antibodies indicated that the observed reduced activity was not a result of reduced protein levels (Fig. 2C). It is likely that a conformational change induced by this deletion prevented this construct from being as transcriptionally active as the other N-terminal deletion construct. In contrast, transcriptional activation of the ftz mTATA reporter was not significantly affected by co-transfection of the aa 95–135 deletion construct (Fig. 2B). Hence, the N terminus of Caudal may be dispensable for the core promoter-preferential activation of ftz by Caudal.
Interestingly, reduced ftz mTATA reporter activity was observed upon co-transfection of a Caudal expression construct in which aa 363–427 were deleted, whereas deletion of aa 399–420 did not significantly reduce the preferential activation (Fig. 2D). Taken together, the results suggest that the C-terminal region of Caudal contributes to the DPE-preferential activation of ftz by Caudal.
The Region Containing Both the Homeodomain and the C Terminus of Caudal Is Sufficient to Confer Core Promoter-preferential Activation to a Heterologous DNA-binding Domain
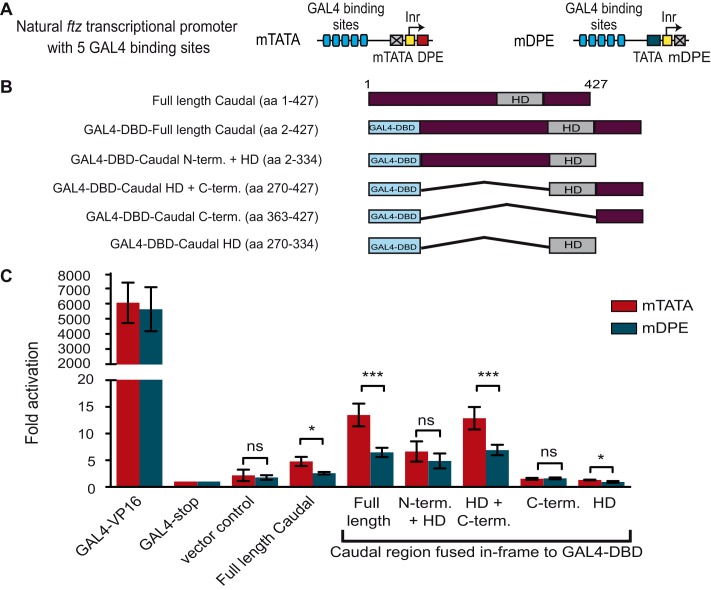
To examine which region of Caudal is sufficient to confer core promoter-preferential activation to a heterologous DNA-binding domain, we utilized the GAL4 luciferase-based assay in which the GAL4-DBD was either fused to the N terminus and the HD of Caudal (aa 2–334), the HD and the C terminus of Caudal (aa 270–427), the C-terminal region whose deletion resulted in reduced preferential activation (aa 363–427; Fig. 2D), or the HD of Caudal (aa 270–334) (Fig. 3). Full-length Caudal as well as the VP16 transcriptional activation domain were fused to GAL4 as positive controls. The GAL4 followed by a stop codon and the full-length Caudal that was not fused to GAL4-DBD were used as negative controls. Each GAL4 fusion was subcloned into the pAc5.1 expression vector. The reporter activities of S2R+ cells that were co-transfected with a firefly luciferase reporter driven by five GAL4-binding sites upstream of ftz minimal promoter (containing either the mTATA or mDPE) (Fig. 3A) as well as with the various GAL4-Caudal expression plasmids (Fig. 3B) were analyzed using Dual-Luciferase assays (Fig. 3C).
FIGURE 3.
The region containing the homeodomain and the C terminus of Caudal is sufficient to convey core promoter-preferential activation. A, schematic representation of the DNA fragments that drive firefly luciferase expression and contain five GAL4-binding sites upstream of minimal ftz promoter containing either an mTATA or mDPE motif. B, schematic representation of the GAL4 DNA-binding domain fused to various regions of Caudal. C, Drosophila S2R+ cells were co-transfected with the indicated GAL4 DNA-binding domain fusion protein expression vector as well as a firefly luciferase reporter gene driven by five GAL4 sites upstream of a ftz promoter containing either an mTATA or mDPE motif. To normalize for variations in transfection efficiency, cells were co-transfected with a Pol III-Renilla luciferase control plasmid. Cell extracts were assayed for Dual-Luciferase activity. The activities are reported relative to the activity of promoters that were co-transfected with the GAL4-stop expression plasmid, which were defined to be 1. The graph represents an average of three to six independent experiments. Error bars represent S.E. ns, p > 0.05; *, 0.01 ≤ p ≤ 0.05; ***, p ≤ 0.001.
As shown in Fig. 3C, the GAL4-DBD fused to the full-length Caudal activates the GAL4-ftz reporters in a core promoter-preferential manner. As expected, the transcriptional activation by the GAL4-VP16 fusion protein was very high compared with the other constructs. Neither the GAL4-stop expression vector nor the pAc5.1 vector control activated the GAL4-ftz reporters. Low transcription levels were observed by co-transfection of Caudal that was not fused to the GAL4-DBD. Nevertheless, the activity observed by this construct was significantly lower than the activity observed for the GAL4-fused full-length Caudal. It is of note that we have previously compared the activity of the untagged Caudal with FLAG-tagged Caudal and observed no differences. The GAL4-DBD fusion protein containing the N-terminal and the HD regions of Caudal showed moderate transcriptional activity; albeit no preference for a particular core promoter motif was observed. Interestingly, the GAL4 fusion of the region containing the HD and the C terminus of Caudal showed marked preferential activity, whereas the C terminus of Caudal alone did not confer preferential activation to the GAL4 fusion protein. The HD of Caudal displayed preferential activation, but the overall transcription levels were very low, very similar to the vector control or the GAL4-stop vector. Hence, although the C terminus is important for DPE preferential activation, only in the presence of the HD can it confer preferential transcriptional activation to the heterologous GAL4-DBD.
The Regions Containing Both the Homeodomains and the N Termini of Vertebrate Cdx1 and Cdx2 Are Sufficient to Confer Core Promoter-preferential Activation to a Heterologous DNA-binding Domain
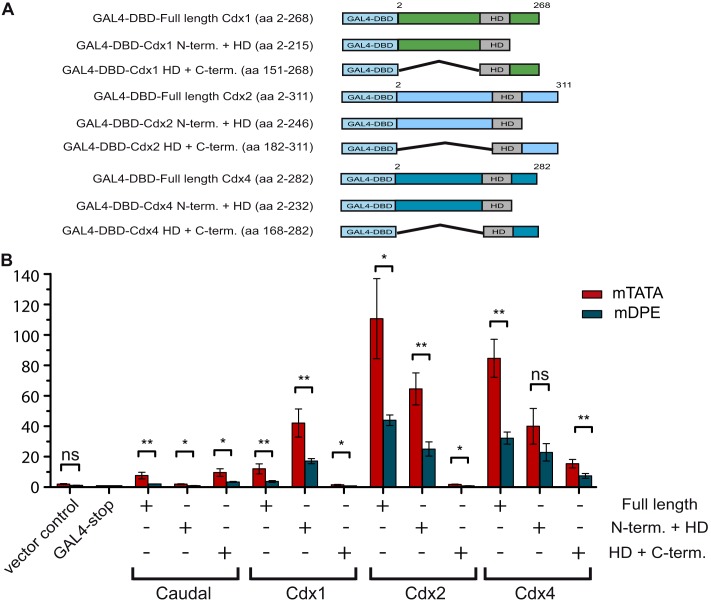
To examine which region of each of the Cdx proteins is sufficient to confer the DPE-preferential activation of ftz, we generated nine GAL4-DBD-Cdx fusion constructs. The Cdx1-GAL4 constructs include the full length (aa 2–268), the N terminus and the HD (aa 2–215), and the HD and C terminus (aa 151–268) of the Cdx1 protein. The Cdx2-GAL4 constructs include the full length (aa 2–311), the N terminus and the HD (aa 2–246), and the HD and C terminus (aa 182–311) of the Cdx2 protein. The GAL4-Cdx4 constructs include the full length (aa 2–282), the N terminus and the HD (aa 2–232), and the HD and C terminus (aa 168–282) of the Cdx4 protein (Fig. 4A). Consistent with the data presented in Fig. 1, all Cdx proteins activated ftz in a DPE-preferential manner (Fig. 4B). The N termini and the HDs of Cdx1 and Cdx2 proteins were sufficient to confer preferential activation to the GAL4 fusion protein (Fig. 4B). The preferential activation of the reporters by the N terminus and the HD of mCdx4 has a borderline statistical significance (p < 0.0056). It is of note that the C termini of all mCdx proteins displayed preferential activation, but the overall transcription levels, especially by mCdx1 and mCdx2, were low.
FIGURE 4.
The region containing the homeodomain and the N terminus of mouse Cdx1, Cdx2, and Cdx4 is sufficient to convey core promoter-preferential activation. A, schematic representation of the GAL4 DNA-binding domain fused to various regions of Cdx1, Cdx2, and Cdx4. B, Drosophila S2R+ cells were co-transfected with the indicated GAL4 DNA-binding domain fusion protein expression vector as well as a firefly luciferase reporter gene driven by five GAL4 sites upstream of a ftz promoter containing either an mTATA or mDPE motif. To normalize for variations in transfection efficiency, cells were co-transfected with a Pol III-Renilla luciferase control plasmid. Cell extracts were assayed for Dual-Luciferase activity. The activities are reported relative to the activity of promoters that were co-transfected with the GAL4-stop expression plasmid, which were defined to be 1. The graph represents an average of three independent experiments. Error bars represent S.E. ns, p > 0.05; *, 0.01 ≤ p ≤ 0.05; **, 0.001 ≤ p ≤ 0.01.
Drosophila CBP Is a Co-activator for Caudal-regulated Transcriptional Activation of the ftz Reporter
Because Caudal-binding sites are located hundreds of base pairs upstream of the activated promoter, we wanted to identify proteins that might mediate between Caudal binding at the enhancer and the core promoter region. The Caudal-binding sites upstream of the ftz promoter have previously been mapped by DNase I footprinting (45).
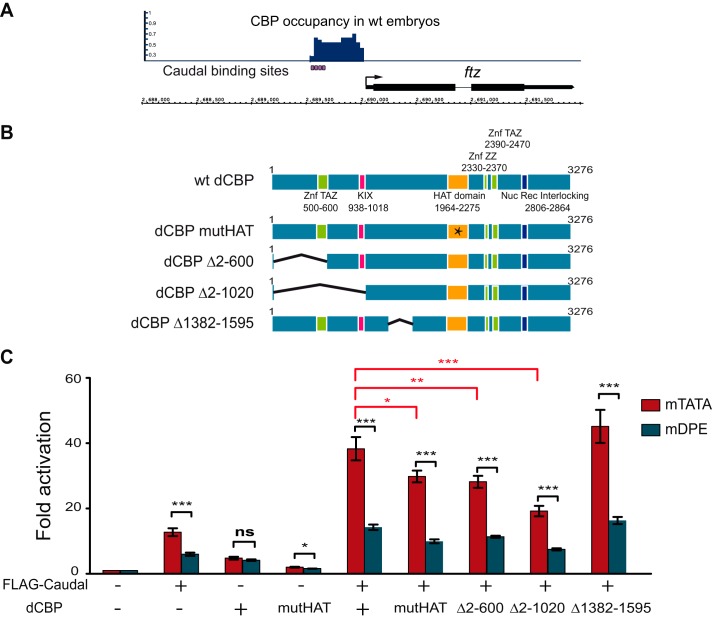
Mouse Cdx2 shows functional similarity to Drosophila Caudal in preferentially regulating ftz expression (Fig. 1C). It has previously been demonstrated that Cdx2 makes direct protein-protein interaction with the N-terminal domain of p300 (72). Notably, Drosophila only has one p300/CBP ortholog, named dCBP or nejire. ChIP analysis of Drosophila embryos using dCBP antibodies (73) revealed a cluster of dCBP-binding sites at the enhancer region of the ftz gene that overlap the previously characterized Caudal-binding sites in the ftz enhancer (Fig. 5A). Thus, we hypothesized that dCBP may serve as a co-activator for the core promoter-preferential activation exhibited by Caudal.
FIGURE 5.
Drosophila CBP co-activates Caudal-regulated preferential transcriptional activation of the ftz reporter via the N terminus of CBP as well as the HAT domain. A, CBP occupancy at the ftz genomic locus in WT embryos overlaps previously defined Caudal DNA-binding sites. ChIP-sequencing peaks for CBP in 2–4-h-old WT embryos (73) are shown for the ftz locus. Caudal DNA-binding sites that have previously been characterized using DNase I footprinting (45) are shown below. B, schematic representation of the full-length dCBP protein, the dCBP HAT mutant harboring a point mutation (denoted by a star), and the dCBP mutants harboring internal deletions. C, Drosophila S2R+ cells were co-transfected with firefly luciferase reporter constructs driven by the ftz enhancer-promoter containing either an mTATA or mDPE motif as well as plasmids encoding FLAG-Caudal, dCBP, or the indicated dCBP mutants containing either mutation in HAT domain (mutHAT) or internal deletions (marked by Δ). To normalize for variations in transfection efficiency, cells were co-transfected with a Pol III-Renilla luciferase control plasmid and assayed for Dual-Luciferase activity. The activities are reported relative to the activities of the promoters in the absence of co-transfected Caudal or CBP expression plasmids, which were defined to be 1. The graph represents an average of three to four independent experiments. Error bars represent S.E. ns, p > 0.05; *, 0.01 ≤ p ≤ 0.05; **, 0.001 ≤ p ≤ 0.01; ***, p ≤ 0.001. ZnF, zinc finger; Nuc Rec, nuclear receptor coactivator.
To test the ability of dCBP to co-activate Caudal regulated transcriptional activation of the ftz mTATA and mDPE reporters, we used transient transfection assays in S2R+ cells. Cells were co-transfected with Caudal as well as either wild-type dCBP, a previously characterized dCBP HAT domain mutant (F2161A point mutation) (62), or one of three dCBP deletion mutants, each cloned into the pAc5.1 expression vector (depicted in Fig. 5B). As can be seen in Fig. 5C, wild-type dCBP co-activated the Caudal-regulated transcriptional activation of the ftz reporter about 3-fold relative to Caudal-mediated ftz activation. Notably, S2R+ cells express moderate levels of endogenous dCBP (FlyBase), and it is possible that the activation observed by transfected Caudal in the absence of co-transfected dCBP already involves co-activation by endogenous dCBP. The mutated HAT dCBP was able to co-activate the Caudal-regulated transactivation of the ftz mTATA reporter albeit to a lower degree, suggesting that the HAT domain contributes to the DPE-specific transcription mediated by Caudal. Based on the previously published protein-protein interaction between Cdx2 and the N-terminal domain of p300 (72), we examined whether the N-terminal region of dCBP might be involved in co-activating Caudal-mediated transcription. To that end, we constructed two N-terminal deletion mutants: dCBP Δ2–600 in which the transcriptional adaptor zinc-binding (TAZ) domain has been deleted, and dCBP Δ2–1020 in which both the TAZ and KIX domains have been deleted (Fig. 5B). The TAZ and KIX domains were shown to be important for protein-protein interactions (for reviews, see Refs. 74–76). Deletion of amino acids 2–600 reduced dCBP co-activation, and deletion of amino acids 2–1020 further reduced dCBP co-activation (Fig. 5C). To examine whether the co-activation of Caudal lies in the N terminus of dCBP (or whether any deletion in dCBP will cause a reduction in co-activation), we generated an additional dCBP deletion mutant in which amino acids 1382–1595 were deleted (dCBP Δ1382–1595). As expected, this mutant was able to co-activate Caudal (Fig. 5C). Taken together, these results demonstrate that dCBP co-activates Caudal-mediated transcription of the ftz reporter via the DPE motif and that N terminus of dCBP is important for this co-activation.
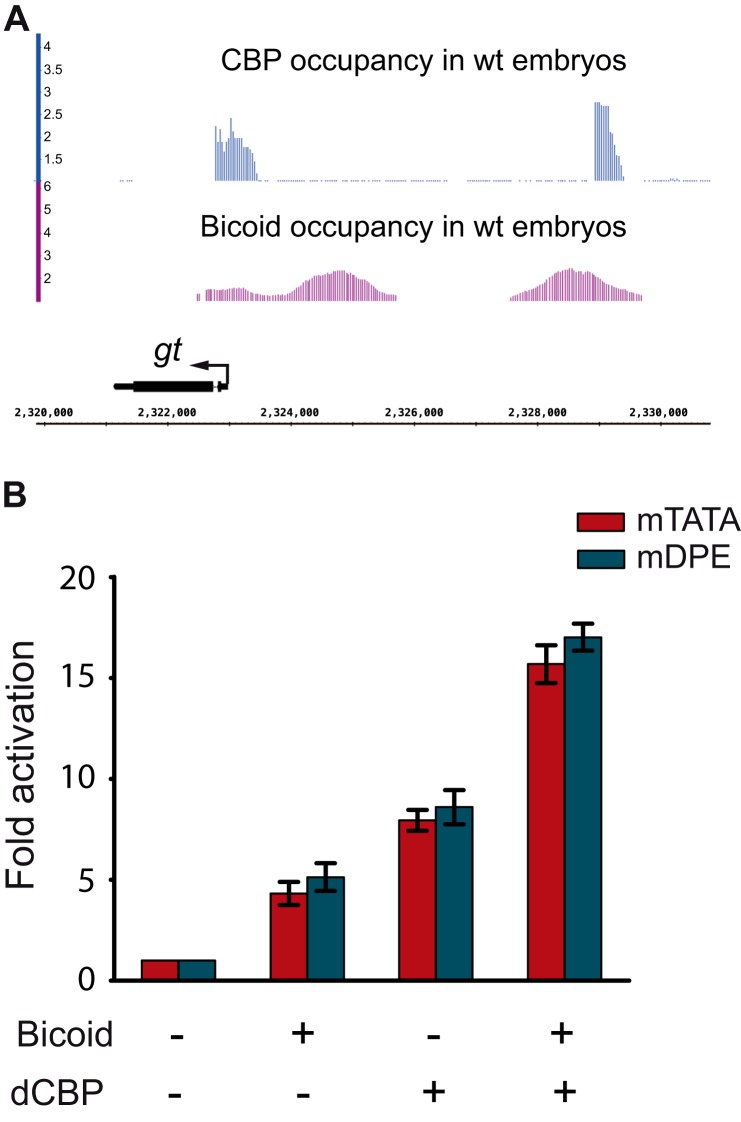
We next wanted to examine whether dCBP can provide another homeodomain transcription factor, such as Bicoid, the ability to activate transcription with a distinct preference for a DPE motif. dCBP has previously been shown to function as a Bicoid co-activator in S2 cells (77, 78). giant (gt) is a Bicoid target gene (79–81) whose core promoter contains a TATA box, an Inr, and a DPE motif (38). ChIP analysis of Drosophila embryos using dCBP antibodies (73) revealed dCBP-binding sites that overlap the ChIP-chip peaks for Bicoid (82) in the gt enhancer (Fig. 6A).
FIGURE 6.
Drosophila CBP co-activates Bicoid-regulated transcriptional activation of the gt reporter without a preference for a TATA box or a DPE motif. A, CBP and Bicoid occupancy at the gt locus in WT embryos. ChIP-sequencing peaks for CBP (73) and ChIP-chip peaks for Bicoid (82) in 2–4-h old WT embryos are shown for the gt locus. Occupancy is plotted as log2 -fold enrichment over input. B, Drosophila S2R+ cells were co-transfected with gt reporter constructs that contain the gt enhancer and promoter sequences from −2031 to +40 relative to the +1 start site and are identical except for an mTATA or mDPE motif as well as an expression vector for Bicoid and/or dCBP. To normalize for variations in transfection efficiency, cells were co-transfected with a Pol III-Renilla luciferase control plasmid and assayed for Dual-Luciferase activity. The activities are reported relative to the activities of the promoters in the absence of co-transfected Bicoid or dCBP expression plasmids, which were defined to be 1. The graph represents an average of three independent experiments. Error bars represent S.E.
We analyzed co-activation of gt reporter genes by the Bicoid transcription factor using firefly luciferase reporter genes driven by either a DPE-dependent (containing mTATA) or a TATA-dependent (containing mDPE) version of the gt transcriptional control region from −2031 to +40 relative to the RNA start site (83). S2R+ cells were transfected with either a DPE-dependent or a TATA-dependent gt reporter construct as well as an expression vector for Bicoid and/or dCBP. As can be seen in Fig. 6B, although dCBP co-activated Bicoid-regulated gt transcription it did not provide Bicoid with a preference for a core promoter motif. Notably, the activation of the gt reporters by dCBP in the absence of co-transfected Bicoid did not result from activation of endogenous Bicoid as S2R+ do not express bicoid (FlyBase). It likely results from the co-activation of other transcription factors that are expressed in S2R+ cells and activate gt. Taken together, the core promoter-preferential co-activation observed for Caudal-mediated transcription is a combination of intrinsic properties of Caudal and dCBP and not a general feature of dCBP.
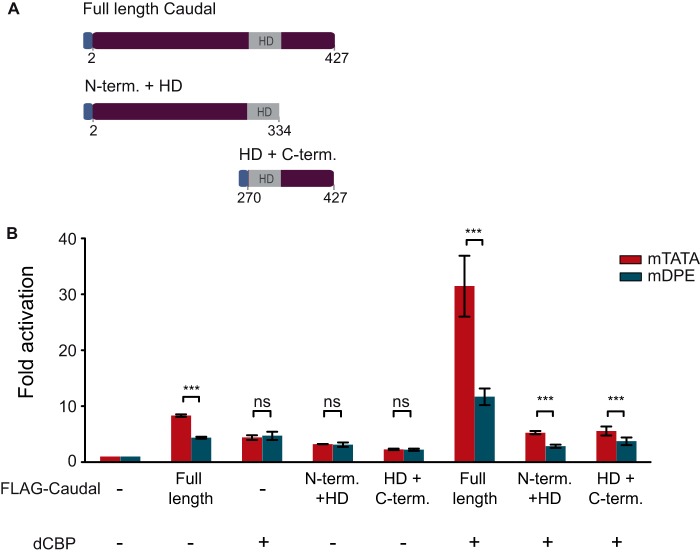
To determine whether dCBP can co-activate a mini-Caudal protein containing either the N terminus and HD or the HD and the C terminus, we co-transfected S2R+ cells with dCBP as well as an expression vector for the N terminus and HD of Caudal or the HD and the C terminus of Caudal (depicted in Fig. 7A). Transfection of either mini-Caudal protein did not result in preferential activation of the ftz reporter (Fig. 7B). The fact that a Caudal protein with a deletion of aa 2–268 activated transcription in a core promoter-preferential manner (Fig. 2B) whereas a mini Caudal protein containing aa 270–427 (HD + C-term.) did not (Fig. 7B) suggests that Lys-269, which is only a few aa away from the HD sequence (spanning aa 275–332 of Drosophila Caudal), participates in DNA binding.
FIGURE 7.
Drosophila CBP can convey preferential transcriptional activation of the ftz reporter to both the N terminus and the C terminus of Caudal. A, schematic representation of the full-length FLAG-Caudal protein and the mini-Caudal proteins containing the N terminus and homeodomain of Caudal (N-term. + HD; aa 2–334) or the homeodomain and C terminus of Caudal (HD + C-term.; aa 270–427). The blue box at the N terminus of the Caudal expression constructs denotes a FLAG tag. B, Drosophila S2R+ cells were co-transfected with ftz reporter constructs containing either an mTATA or a mDPE motif as well as plasmids encoding dCBP, FLAG-tagged full-length-Caudal, or a mini-Caudal protein as depicted. To normalize for variations in transfection efficiency, cells were co-transfected with a Pol III-Renilla luciferase control plasmid and assayed for Dual-Luciferase activity. The activities are reported relative to the activities of the promoters in the absence of co-transfected Caudal or dCBP expression plasmids, which were defined to be 1. The graph represents an average of three independent experiments. Error bars represent S.E. ns, p > 0.05; ***, p ≤ 0.001.
Strikingly, co-transfection of dCBP was able to confer the ability to preferentially activate the DPE-dependent ftz reporter to each of the mini-Caudal proteins. Hence, it is the unique combination of dCBP and Caudal that enables the co-activation of the ftz in a core promoter-preferential manner.
Discussion
The Ability of Drosophila Caudal to Preferentially Activate DPE Transcription Is Conserved to Mouse Cdx Proteins
The vertebrate Cdx genes (Cdx1, Cdx2, and Cdx4) are related to Drosophila Caudal, and their gene products have conserved the ancestral ability to specify the posterior development of the embryo and pattern the anterior-posterior axis. A preference for activators to work with a TATA box, an Inr, or both a TATA box and an Inr has previously been demonstrated using synthetic core promoters (84). We examined the transcriptional activation of the natural ftz promoter in Drosophila Schneider S2R+ cells and discovered that the mouse Cdx1, Cdx2, and Cdx4 transcription factors have the ability to preferentially activate DPE-dependent promoters. These findings imply that the core promoter composition plays a role in transcriptional regulation of gene expression in vertebrates. It remains to be determined which of the mouse target genes of the Cdx family of transcription factors are preferentially activated via the DPE. The Cdx proteins are master regulators of Hox gene expression (55, 58, 59, 61, 85–87). Further characterization of the molecular mechanism governing transcriptional activation by the vertebrate Cdx proteins via the core promoter will advance our understanding of the regulation of Hox gene expression by Cdx family transcription factors.
Core Promoter-preferential Activation of ftz Is Mediated via the Homeodomain and C Terminus of Drosophila Caudal and the Homeodomain and N Termini of the Cdx Proteins
Caudal has previously been shown to preferentially activate transcription of its target ftz through the DPE motif, but the mechanism by which Caudal discriminates between different core promoters was unclear. Here, we discovered that a region containing the C terminus of Caudal was essential for core promoter-specific activation of ftz and that a region containing both the Drosophila Caudal HD and the C terminus was sufficient to confer core promoter-preferential activation to GAL4-DBD. Similarly, vertebrate Cdx1, Cdx2, and Cdx4 conferred preferential activation to the GAL4-DBD. Notably, the Caudal HD region was unable to mediate core-promoter specific activation when fused to the N-terminal region. Accordingly, not all HD-containing transcription factors possessed such core promoter-preferential activation, and Caudal was unique in its ability. Hence, the HD, in addition to DNA binding, might provide a unique moiety that works in concert with the C terminus of Drosophila Caudal to enable core promoter-preferential activation. It remains to be determined whether transcription factors that bear similarity to the HD and C terminus of Caudal possess core promoter-preferential capabilities.
Drosophila CBP Co-activates Caudal-regulated Preferential Activation of ftz
Because the regulation of ftz by Caudal involves the interaction of Caudal with an enhancer region located hundreds of base pairs away from the promoter, we hypothesized that there is a co-activator that serves as a core promoter-specific mediator between the enhancer and promoter. We discovered that dCBP co-activates Caudal-mediated DPE-preferential activation of the ftz enhancer-promoter. Furthermore, we demonstrated that both the HAT activity and the N terminus of dCBP contribute to the DPE-preferential activation. The N terminus of dCBP contains both the TAZ and KIX domains of dCBP that have been shown to interact with multiple transcription factors. Hence, it is possible that the N terminus of dCBP also interacts with Caudal. Moreover, the acetyltransferase activity of dCBP is not the only determining factor in co-activation of the ftz Caudal target gene. We have further demonstrated that dCBP does not confer core promoter-preferential activation to Bicoid-mediated transcription. Taken together, our data shed light on the mechanism of core promoter-specific transcriptional regulation by Caudal.
Remarkably, dCBP has been shown to preferentially co-occupy genomic regions with the Dorsal transcription factor that is a key regulator of dorsal-ventral patterning. We have previously shown that the DPE is a transcriptional element shared by many Dorsal target genes (88). It remains to be determined whether co-activation of Dorsal targets by dCBP is influenced by the core promoter composition.
Author Contributions
H. S.-S., J. S., D. I., and T. J.-G. conceived the study and wrote the paper. H. S.-S., J. S., D. I., and T. J.-G. designed the constructs, performed experiments, and analyzed the results shown in Figs. 1–5 and 7. M. F. designed the constructs used in Fig. 2. A. O.-S. performed the experiments shown in Fig. 6B. M. M. provided ChIP-sequencing analysis data shown in Figs. 5A and 6A. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Doron Ginsberg, Anna Sloutskin, Yehuda Danino, and Adi Kedmi for critical reading of the manuscript. We thank Rachel Levy-Drummer for invaluable assistance with statistical analysis. We thank David Lohnes (University of Ottawa), Sarah Smolik (Oregon Health and Science University), and Norbert Perrimon (Harvard Medical School) for the generous gift of reagents. We thank Jim Kadonaga (University of California, San Diego) for invaluable suggestions. We thank Yarden Opatowski, Benny Motro, Shani Basch-Barzilay, and Yonathan Zehavi (Bar-Ilan University) for advice and assistance.
This work was supported by Israel Science Foundation Grant 798/10 (to T. J.-G) and the European Union Seventh Framework Programme (Marie Curie International Reintegration Grant 256491 to T.J.-G.). The analysis of the transcriptional activity of Bicoid was supported by United States-Israel Binational Science Foundation Grant 2009428 (to T. J.-G. and James T. Kadonaga). The authors declare that they have no conflicts of interest with the contents of this article.
- Pol
- polymerase
- TSS
- transcription start site
- DPE
- downstream core promoter element
- mCdx
- mouse Cdx
- ftz
- fushi tarazu
- dCBP
- Drosophila CBP
- CBP
- CREB-binding protein
- CREB
- cAMP-response element-binding protein
- Inr
- initiator
- Cdx
- Caudal-related homeobox
- aa
- amino acids
- term.
- terminus
- HD
- homeodomain
- mTATA
- mutated TATA box
- mDPE
- mutated DPE
- HAT
- histone acetyltransferase
- DBD
- DNA-binding domain
- TAZ
- transcriptional adaptor zinc-binding
- KIX
- kinase-inducible domain interacting
- gt
- giant.
References
- 1. Lagha M., Bothma J. P., Levine M. (2012) Mechanisms of transcriptional precision in animal development. Trends Genet. 28, 409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levine M., Cattoglio C., Tjian R. (2014) Looping back to leap forward: transcription enters a new era. Cell 157, 13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levine M., Davidson E. H. (2005) Gene regulatory networks for development. Proc. Natl. Acad. Sci. U.S.A. 102, 4936–4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levine M., Tjian R. (2003) Transcription regulation and animal diversity. Nature 424, 147–151 [DOI] [PubMed] [Google Scholar]
- 5. Pearson J. C., Lemons D., McGinnis W. (2005) Modulating Hox gene functions during animal body patterning. Nat. Rev. Genet. 6, 893–904 [DOI] [PubMed] [Google Scholar]
- 6. Calo E., Wysocka J. (2013) Modification of enhancer chromatin: what, how, and why? Mol. Cell 49, 825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clapier C. R., Cairns B. R. (2009) The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 78, 273–304 [DOI] [PubMed] [Google Scholar]
- 8. Spitz F., Furlong E. E. (2012) Transcription factors: from enhancer binding to developmental control. Nat. Rev. Genet. 13, 613–626 [DOI] [PubMed] [Google Scholar]
- 9. Tee W. W., Reinberg D. (2014) Chromatin features and the epigenetic regulation of pluripotency states in ESCs. Development 141, 2376–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dikstein R. (2011) The unexpected traits associated with core promoter elements. Transcription 2, 201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heintzman N. D., Ren B. (2007) The gateway to transcription: identifying, characterizing and understanding promoters in the eukaryotic genome. Cell. Mol. Life Sci. 64, 386–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Juven-Gershon T., Hsu J.-Y., Theisen J. W., Kadonaga J. T. (2008) The RNA polymerase II core promoter—the gateway to transcription. Curr. Opin. Cell Biol. 20, 253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Juven-Gershon T., Kadonaga J. T. (2010) Regulation of gene expression via the core promoter and the basal transcriptional machinery. Dev. Biol. 339, 225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kadonaga J. T. (2012) Perspectives on the RNA polymerase II core promoter. Wiley Interdiscip. Rev. Dev. Biol. 1, 40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lenhard B., Sandelin A., Carninci P. (2012) Metazoan promoters: emerging characteristics and insights into transcriptional regulation. Nat. Rev. Genet. 13, 233–245 [DOI] [PubMed] [Google Scholar]
- 16. Ohler U., Wassarman D. A. (2010) Promoting developmental transcription. Development 137, 15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smale S. T. (2001) Core promoters: active contributors to combinatorial gene regulation. Genes Dev. 15, 2503–2508 [DOI] [PubMed] [Google Scholar]
- 18. Smale S. T., Kadonaga J. T. (2003) The RNA polymerase II core promoter. Annu. Rev. Biochem. 72, 449–479 [DOI] [PubMed] [Google Scholar]
- 19. Fuda N. J., Ardehali M. B., Lis J. T. (2009) Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature 461, 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sikorski T. W., Buratowski S. (2009) The basal initiation machinery: beyond the general transcription factors. Curr. Opin. Cell Biol. 21, 344–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas M. C., Chiang C. M. (2006) The general transcription machinery and general cofactors. Crit. Rev. Biochem. Mol. Biol. 41, 105–178 [DOI] [PubMed] [Google Scholar]
- 22. Goldberg M. L. (1979) Sequence analysis of Drosophila histone genes. Ph.D. thesis, Stanford University [Google Scholar]
- 23. Smale S. T., Baltimore D. (1989) The “initiator” as a transcription control element. Cell 57, 103–113 [DOI] [PubMed] [Google Scholar]
- 24. Parry T. J., Theisen J. W., Hsu J.-Y., Wang Y.-L., Corcoran D. L., Eustice M., Ohler U., Kadonaga J. T. (2010) The TCT motif, a key component of an RNA polymerase II transcription system for the translational machinery. Genes Dev. 24, 2013–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lim C. Y., Santoso B., Boulay T., Dong E., Ohler U., Kadonaga J. T. (2004) The MTE, a new core promoter element for transcription by RNA polymerase II. Genes Dev. 18, 1606–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Theisen J. W., Lim C. Y., Kadonaga J. T. (2010) Three key subregions contribute to the function of the downstream RNA polymerase II core promoter. Mol. Cell. Biol. 30, 3471–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burke T. W., Kadonaga J. T. (1996) Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes Dev. 10, 711–724 [DOI] [PubMed] [Google Scholar]
- 28. Burke T. W., Kadonaga J. T. (1997) The downstream core promoter element, DPE, is conserved from Drosophila to humans and is recognized by TAF(II)60 of Drosophila. Genes Dev. 11, 3020–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kutach A. K., Kadonaga J. T. (2000) The downstream promoter element DPE appears to be as widely used as the TATA box in Drosophila core promoters. Mol. Cell. Biol. 20, 4754–4764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reeve J. N. (2003) Archaeal chromatin and transcription. Mol. Microbiol. 48, 587–598 [DOI] [PubMed] [Google Scholar]
- 31. Carninci P., Sandelin A., Lenhard B., Katayama S., Shimokawa K., Ponjavic J., Semple C. A., Taylor M. S., Engström P. G., Frith M. C., Forrest A. R., Alkema W. B., Tan S. L., Plessy C., Kodzius R., Ravasi T., Kasukawa T., Fukuda S., Kanamori-Katayama M., Kitazume Y., Kawaji H., Kai C., Nakamura M., Konno H., Nakano K., Mottagui-Tabar S., Arner P., Chesi A., Gustincich S., Persichetti F., Suzuki H., Grimmond S. M., Wells C. A., Orlando V., Wahlestedt C., Liu E. T., Harbers M., Kawai J., Bajic V. B., Hume D. A., Hayashizaki Y. (2006) Genome-wide analysis of mammalian promoter architecture and evolution. Nat. Genet. 38, 626–635 [DOI] [PubMed] [Google Scholar]
- 32. Ponjavic J., Lenhard B., Kai C., Kawai J., Carninci P., Hayashizaki Y., Sandelin A. (2006) Transcriptional and structural impact of TATA-initiation site spacing in mammalian core promoters. Genome Biol. 7, R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burley S. K., Roeder R. G. (1996) Biochemistry and structural biology of transcription factor IID (TFIID). Annu. Rev. Biochem. 65, 769–799 [DOI] [PubMed] [Google Scholar]
- 34. FitzGerald P. C., Sturgill D., Shyakhtenko A., Oliver B., Vinson C. (2006) Comparative genomics of Drosophila and human core promoters. Genome Biol. 7, R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gershenzon N. I., Trifonov E. N., Ioshikhes I. P. (2006) The features of Drosophila core promoters revealed by statistical analysis. BMC Genomics 7, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ohler U., Liao G. C., Niemann H., Rubin G. M. (2002) Computational analysis of core promoters in the Drosophila genome. Genome Biol. 3, RESEARCH0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chalkley G. E., Verrijzer C. P. (1999) DNA binding site selection by RNA polymerase II TAFs: a TAF(II)250-TAF(II)150 complex recognizes the Initiator. EMBO J. 18, 4835–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Juven-Gershon T., Hsu J.-Y., Kadonaga J. T. (2008) Caudal, a key developmental regulator, is a DPE-specific transcriptional factor. Genes Dev. 22, 2823–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoey T., Doyle H. J., Harding K., Wedeen C., Levine M. (1986) Homeo box gene expression in anterior and posterior regions of the Drosophila embryo. Proc. Natl. Acad. Sci. U.S.A. 83, 4809–4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Levine M., Harding K., Wedeen C., Doyle H., Hoey T., Radomska H. (1985) Expression of the homeo box gene family in Drosophila. Cold Spring Harb. Symp. Quant. Biol. 50, 209–222 [DOI] [PubMed] [Google Scholar]
- 41. Macdonald P. M., Struhl G. (1986) A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature 324, 537–545 [DOI] [PubMed] [Google Scholar]
- 42. Mlodzik M., Fjose A., Gehring W. J. (1985) Isolation of caudal, a Drosophila homeo box-containing gene with maternal expression, whose transcripts form a concentration gradient at the pre-blastoderm stage. EMBO J. 4, 2961–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mlodzik M., Gehring W. J. (1987) Expression of the caudal gene in the germ line of Drosophila: formation of an RNA and protein gradient during early embryogenesis. Cell 48, 465–478 [DOI] [PubMed] [Google Scholar]
- 44. Mlodzik M., Gibson G., Gehring W. J. (1990) Effects of ectopic expression of caudal during Drosophila development. Development 109, 271–277 [DOI] [PubMed] [Google Scholar]
- 45. Dearolf C. R., Topol J., Parker C. S. (1989) The caudal gene product is a direct activator of fushi tarazu transcription during Drosophila embryogenesis. Nature 341, 340–343 [DOI] [PubMed] [Google Scholar]
- 46. Chawengsaksophak K., de Graaff W., Rossant J., Deschamps J., Beck F. (2004) Cdx2 is essential for axial elongation in mouse development. Proc. Natl. Acad. Sci. U.S.A. 101, 7641–7645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chawengsaksophak K., James R., Hammond V. E., Köntgen F., Beck F. (1997) Homeosis and intestinal tumours in Cdx2 mutant mice. Nature 386, 84–87 [DOI] [PubMed] [Google Scholar]
- 48. Copf T., Schröder R., Averof M. (2004) Ancestral role of caudal genes in axis elongation and segmentation. Proc. Natl. Acad. Sci. U.S.A. 101, 17711–17715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Edgar L. G., Carr S., Wang H., Wood W. B. (2001) Zygotic expression of the caudal homolog pal-1 is required for posterior patterning in Caenorhabditis elegans embryogenesis. Dev. Biol. 229, 71–88 [DOI] [PubMed] [Google Scholar]
- 50. Epstein M., Pillemer G., Yelin R., Yisraeli J. K., Fainsod A. (1997) Patterning of the embryo along the anterior-posterior axis: the role of the caudal genes. Development 124, 3805–3814 [DOI] [PubMed] [Google Scholar]
- 51. Hunter C. P., Kenyon C. (1996) Spatial and temporal controls target pal-1 blastomere-specification activity to a single blastomere lineage in C. elegans embryos. Cell 87, 217–226 [DOI] [PubMed] [Google Scholar]
- 52. Marom K., Shapira E., Fainsod A. (1997) The chicken caudal genes establish an anterior-posterior gradient by partially overlapping temporal and spatial patterns of expression. Mech. Dev. 64, 41–52 [DOI] [PubMed] [Google Scholar]
- 53. Meyer B. I., Gruss P. (1993) Mouse Cdx-1 expression during gastrulation. Development 117, 191–203 [DOI] [PubMed] [Google Scholar]
- 54. Moreno E., Morata G. (1999) Caudal is the Hox gene that specifies the most posterior Drosophila segment. Nature 400, 873–877 [DOI] [PubMed] [Google Scholar]
- 55. Subramanian V., Meyer B. I., Gruss P. (1995) Disruption of the murine homeobox gene Cdx1 affects axial skeletal identities by altering the mesodermal expression domains of Hox genes. Cell 83, 641–653 [DOI] [PubMed] [Google Scholar]
- 56. van den Akker E., Forlani S., Chawengsaksophak K., de Graaff W., Beck F., Meyer B. I., Deschamps J. (2002) Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development 129, 2181–2193 [DOI] [PubMed] [Google Scholar]
- 57. Wolff C., Schröder R., Schulz C., Tautz D., Klingler M. (1998) Regulation of the Tribolium homologues of caudal and hunchback in Drosophila: evidence for maternal gradient systems in a short germ embryo. Development 125, 3645–3654 [DOI] [PubMed] [Google Scholar]
- 58. Davidson A. J., Ernst P., Wang Y., Dekens M. P., Kingsley P. D., Palis J., Korsmeyer S. J., Daley G. Q., Zon L. I. (2003) cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature 425, 300–306 [DOI] [PubMed] [Google Scholar]
- 59. Davidson A. J., Zon L. I. (2006) The caudal-related homeobox genes cdx1a and cdx4 act redundantly to regulate hox gene expression and the formation of putative hematopoietic stem cells during zebrafish embryogenesis. Dev. Biol. 292, 506–518 [DOI] [PubMed] [Google Scholar]
- 60. Rawat V. P., Humphries R. K., Buske C. (2012) Beyond Hox: the role of ParaHox genes in normal and malignant hematopoiesis. Blood 120, 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Y., Yabuuchi A., McKinney-Freeman S., Ducharme D. M., Ray M. K., Chawengsaksophak K., Archer T. K., Daley G. Q. (2008) Cdx gene deficiency compromises embryonic hematopoiesis in the mouse. Proc. Natl. Acad. Sci. U.S.A. 105, 7756–7761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ludlam W. H., Taylor M. H., Tanner K. G., Denu J. M., Goodman R. H., Smolik S. M. (2002) The acetyltransferase activity of CBP is required for wingless activation and H4 acetylation in Drosophila melanogaster. Mol. Cell. Biol. 22, 3832–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Faas L., Isaacs H. V. (2009) Overlapping functions of Cdx1, Cdx2, and Cdx4 in the development of the amphibian Xenopus tropicalis. Dev. Dyn. 238, 835–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grainger S., Hryniuk A., Lohnes D. (2013) Cdx1 and Cdx2 exhibit transcriptional specificity in the intestine. PLoS One 8, e54757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Grainger S., Savory J. G., Lohnes D. (2010) Cdx2 regulates patterning of the intestinal epithelium. Dev. Biol. 339, 155–165 [DOI] [PubMed] [Google Scholar]
- 66. Lafontaine C. A., Grainger S., Hess B. L., Béland M., Lohnes D. (2012) Cdx1 interacts physically with a subset of Hox proteins. Biochemistry 51, 9698–9705 [DOI] [PubMed] [Google Scholar]
- 67. Savory J. G., Bouchard N., Pierre V., Rijli F. M., De Repentigny Y., Kothary R., Lohnes D. (2009) Cdx2 regulation of posterior development through non-Hox targets. Development 136, 4099–4110 [DOI] [PubMed] [Google Scholar]
- 68. Savory J. G., Mansfield M., St Louis C., Lohnes D. (2011) Cdx4 is a Cdx2 target gene. Mech. Dev. 128, 41–48 [DOI] [PubMed] [Google Scholar]
- 69. Savory J. G., Pilon N., Grainger S., Sylvestre J. R., Béland M., Houle M., Oh K., Lohnes D. (2009) Cdx1 and Cdx2 are functionally equivalent in vertebral patterning. Dev. Biol. 330, 114–122 [DOI] [PubMed] [Google Scholar]
- 70. Flores M. V., Hall C. J., Davidson A. J., Singh P. P., Mahagaonkar A. A., Zon L. I., Crosier K. E., Crosier P. S. (2008) Intestinal differentiation in zebrafish requires Cdx1b, a functional equivalent of mammalian Cdx2. Gastroenterology 135, 1665–1675 [DOI] [PubMed] [Google Scholar]
- 71. van Nes J., de Graaff W., Lebrin F., Gerhard M., Beck F., Deschamps J. (2006) The Cdx4 mutation affects axial development and reveals an essential role of Cdx genes in the ontogenesis of the placental labyrinth in mice. Development 133, 419–428 [DOI] [PubMed] [Google Scholar]
- 72. Hussain M. A., Habener J. F. (1999) Glucagon gene transcription activation mediated by synergistic interactions of pax-6 and cdx-2 with the p300 co-activator. J. Biol. Chem. 274, 28950–28957 [DOI] [PubMed] [Google Scholar]
- 73. Holmqvist P. H., Boija A., Philip P., Crona F., Stenberg P., Mannervik M. (2012) Preferential genome targeting of the CBP co-activator by Rel and Smad proteins in early Drosophila melanogaster embryos. PLoS Genet. 8, e1002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. De Guzman R. N., Wojciak J. M., Martinez-Yamout M. A., Dyson H. J., Wright P. E. (2005) CBP/p300 TAZ1 domain forms a structured scaffold for ligand binding. Biochemistry 44, 490–497 [DOI] [PubMed] [Google Scholar]
- 75. Radhakrishnan I., Pérez-Alvarado G. C., Parker D., Dyson H. J., Montminy M. R., Wright P. E. (1997) Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell 91, 741–752 [DOI] [PubMed] [Google Scholar]
- 76. Vo N., Goodman R. H. (2001) CREB-binding protein and p300 in transcriptional regulation. J. Biol. Chem. 276, 13505–13508 [DOI] [PubMed] [Google Scholar]
- 77. Fu D., Ma J. (2005) Interplay between positive and negative activities that influence the role of Bicoid in transcription. Nucleic Acids Res. 33, 3985–3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fu D., Wen Y., Ma J. (2004) The co-activator CREB-binding protein participates in enhancer-dependent activities of bicoid. J. Biol. Chem. 279, 48725–48733 [DOI] [PubMed] [Google Scholar]
- 79. Eldon E. D., Pirrotta V. (1991) Interactions of the Drosophila gap gene giant with maternal and zygotic pattern-forming genes. Development 111, 367–378 [DOI] [PubMed] [Google Scholar]
- 80. Kraut R., Levine M. (1991) Spatial regulation of the gap gene giant during Drosophila development. Development 111, 601–609 [DOI] [PubMed] [Google Scholar]
- 81. Rivera-Pomar R., Lu X., Perrimon N., Taubert H., Jäckle H. (1995) Activation of posterior gap gene expression in the Drosophila blastoderm. Nature 376, 253–256 [DOI] [PubMed] [Google Scholar]
- 82. MacArthur S., Li X. Y., Li J., Brown J. B., Chu H. C., Zeng L., Grondona B. P., Hechmer A., Simirenko L., Keränen S. V., Knowles D. W., Stapleton M., Bickel P., Biggin M. D., Eisen M. B. (2009) Developmental roles of 21 Drosophila transcription factors are determined by quantitative differences in binding to an overlapping set of thousands of genomic regions. Genome Biol. 10, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kedmi A., Zehavi Y., Glick Y., Orenstein Y., Ideses D., Wachtel C., Doniger T., Waldman Ben-Asher H., Muster N., Thompson J., Anderson S., Avrahami D., Yates J. R., 3rd, Shamir R., Gerber D., Juven-Gershon T. (2014) Drosophila TRF2 is a preferential core promoter regulator. Genes Dev. 28, 2163–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Emami K. H., Navarre W. W., Smale S. T. (1995) Core promoter specificities of the Sp1 and VP16 transcriptional activation domains. Mol. Cell. Biol. 15, 5906–5916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Charité J., de Graaff W., Consten D., Reijnen M. J., Korving J., Deschamps J. (1998) Transducing positional information to the Hox genes: critical interaction of cdx gene products with position-sensitive regulatory elements. Development 125, 4349–4358 [DOI] [PubMed] [Google Scholar]
- 86. Lohnes D. (2003) The Cdx1 homeodomain protein: an integrator of posterior signaling in the mouse. BioEssays 25, 971–980 [DOI] [PubMed] [Google Scholar]
- 87. Pilon N., Oh K., Sylvestre J. R., Savory J. G., Lohnes D. (2007) Wnt signaling is a key mediator of Cdx1 expression in vivo. Development 134, 2315–2323 [DOI] [PubMed] [Google Scholar]
- 88. Zehavi Y., Kuznetsov O., Ovadia-Shochat A., Juven-Gershon T. (2014) Core promoter functions in the regulation of gene expression of Drosophila dorsal target genes. J. Biol. Chem. 289, 11993–12004 [DOI] [PMC free article] [PubMed] [Google Scholar]