FIGURE 1.
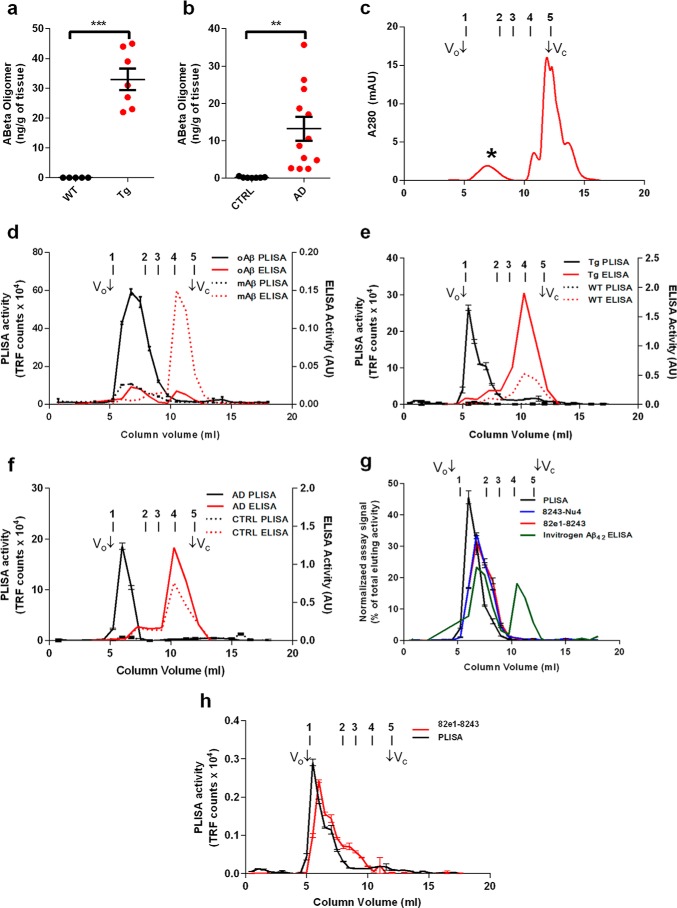
Characterization of PrPC-interacting Aβo. Using PLISA, amounts of PrPC-interacting Aβ oligomers were quantified in brain lysates from wild-type (black dots) and APP/PSEN1 (red dots) transgenic mice (a) as well as from neurologically healthy (black dots) and AD-affected (red dots) human individuals (b). Human AD patient and APP/PSEN mouse samples showed considerably higher levels of Aβo compared with WT mice and human control samples, which had only marginally detectable levels of Aβo. Synthetic Aβ was oligomerized in F-12 medium and separated using size-exclusion chromatography (c). Aβo peak elutes in early fractions (*), whereas monomeric Aβ elutes at the very end of separation (monomeric Aβ peak corresponds to highly absorbing components of F-12 medium, large peak at Vc). SEC fractionation was performed on oligomeric (oAβ, straight lines) and monomeric preparations (mAβ, dashed lines) of synthetic Aβ (d), and fractions were assayed using PLISA (black lines) and conventional ELISAs (red lines). PLISA demonstrated strong preference toward high molecular weight Aβ assemblies showing strong peak in column void volume (Vo) and early fractions, particularly prominent in oligomeric preparation. ELISA was mostly specific toward monomeric forms of Aβ resulting in sharp peak close to fractions corresponding to total column volume (Vc). In analogy with synthetic Aβ preparations, SEC fractionation coupled to PLISA and ELISA was performed on TBS brain lysates of APP/PSEN1 (Tg, straight lines) and wild-type control (WT, dashed lines) mice (e) as well as TBS lysates from post-mortem brain tissue from human AD patients (AD, straight lines) and neurologically healthy control individuals (CTRL, dashed lines) (f). Similarly to synthetic material, PLISA was highly selective toward HMW Aβ assemblies only in AD-related but not in control samples, whereas ELISA was mostly detecting monomeric Aβ. PLISA activity forms a sharp peak in HMW fractions, whereas the activity of Aβ oligomer-specific ELISAs (8243-Nu4, 82e1-8243) distributes proportionally to the amount of eluting Aβo (g). Specificity of PLISA toward HMW Aβo was also true for TBS brain lysates from old APP/PSEN1 mice, whereas 82e1-8243 detected a broader range of Aβo species (h). Bio-Rad Gel Filtration protein standards were used to aid with the molecular weight determination of Aβ species: peak 1, thyroglobulin (bovine), 670 kDa; peak 2, γ-globulin (bovine), 158 kDa; peak 3, ovalbumin (chicken), 44 kDa; peak 4, myoglobin (horse), 17 kDa; peak 5, vitamin B12, 1.35 kDa. mAU, milliabsorbance units. Error bars represent S.E.