FIGURE 1.
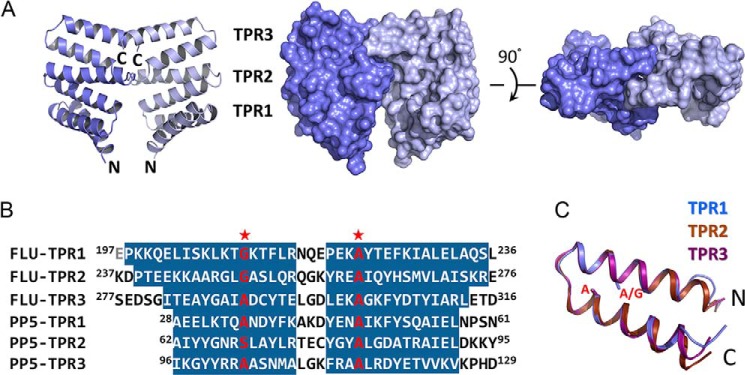
Structure of FLUTPR at 1. 45 Å. A, left, a ribbon representation showing overall structure of FLUTPR dimer. One protomer is colored indigo, and the other is light blue. Middle and right, two perpendicular views in surface representation. B, alignment of TPR motifs from FLU and protein phosphatase 5 (PP5). Residues in α-helix are highlighted. Glu197 is colored gray because it is not observed in the FLUTPR structure. Red stars indicate the strictly conserved residues in TPR motif. C, superimposition of the three TPR motifs. The individual motifs are color-coded. Side chains of residues at the two strictly conserved positions are shown as sticks.
