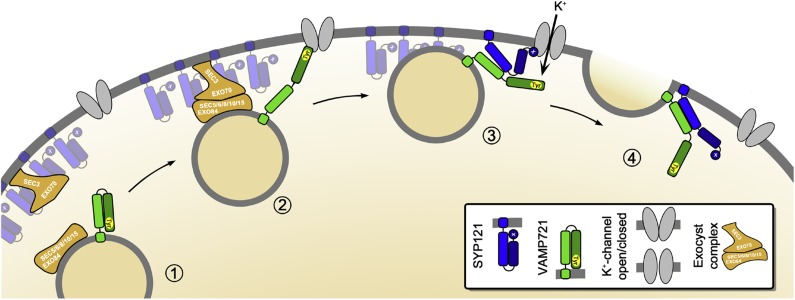
Figure 13.
Vesicle Tethering, Docking, and Fusion Associated with K+ Channel Binding and Control by SYP121 and Its Cognate R-SNAREs VAMP721 and VAMP722.
The hypothetical sequence brings together our understanding of the exocyst in tethering in Arabidopsis (Fendrych et al., 2010), of the channels as focal points for vesicle traffic (Sutter et al., 2006, 2007), and of SYP121 recruitment by the K+ channels from larger “icebergs” of the Qa-SNARE at the plasma membrane (Sieber et al., 2007; Murray and Tamm, 2009; Honsbein et al., 2011). Here vesicle approach (1) and tethering by the exocyst complex leads to docking when VAMP721 binds with the K+ channel (2) to bring the vesicle within 8 to 10 nm of the plasma membrane surface. Once docked via the VAMP longing domain, the K+ channel-VAMP complex recruits SYP121 leading to an exchange of binding (3). Not shown is the anticipated exchange in SYP121 binding between SEC11 and the K+ channel (Karnik et al., 2013b). These events concurrently promote K+ channel activation (3) and bring the SNAREs together for their assembly in the core complex to drive the final stages of membrane fusion (4).