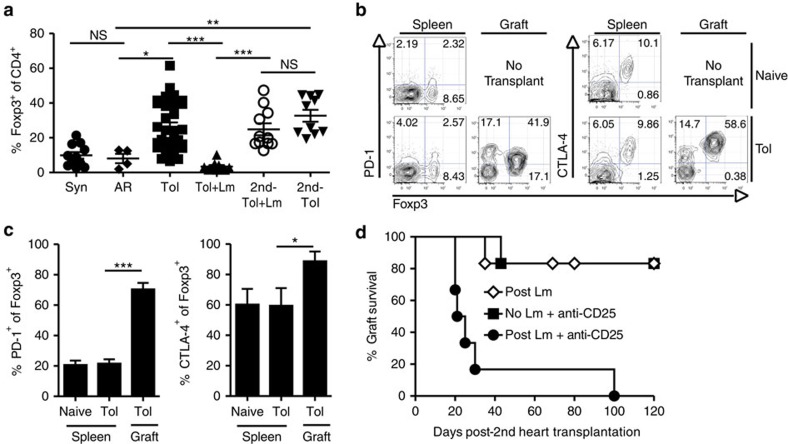
Figure 3. The return of tolerance is dependent on Tregs.
(a) Cardiac allografts were harvested >60 days post transplantation for syngeneic grafts (Syn, n=11) or allogeneic tolerated grafts (Tol, n=34). Acute rejection (AR) allografts (n=4) were harvested 1 week post transplantation. Loss of tolerance hearts were harvested 1 week post Lm (with infection at 60 days post transplant; Tol+Lm, n=10). For the restoration of tolerance, second donor cardiac allografts were transplanted 14 days after Lm infection of tolerant recipients, that is, 1 week post rejection of the first allograft (second-Tol+Lm, n=11). Control second donor allografts were transplanted in tolerant non-infected mice (second-Tol, n=10) at a comparable time (74 days post first heart transplant). Second grafts were harvested 2 weeks after transplantation. Graft-infiltrating leukocytes were analysed by flow cytometry, gated on CD4+ events and displayed as % Foxp3+ of CD4+ cells. Data are presented as mean±s.e.m. and are pooled from 2 to 17 replicate experiments. (b,c) Representative flow cytometry plots of PD-1 and CTLA-4 expression on Foxp3+ Tregs in the spleen and grafts of naïve, untransplanted and tolerant mice (b) and quantification (c). Naïve spleen n=12, Tol spleen n=9, Tol graft n=12. Data are presented as mean±s.e.m. and are pooled from 7 to 8 experiments. *P<0.05, **P<0.01, ***P<0.001; NS, not significant by one-way analysis of variance with Bonferroni or Kruskal–Wallis with Dunn's for multiple comparisons. (d) Fourteen days after Lm infection, recipients with rejected hearts and uninfected tolerant mice were transplanted with donor-matched heart grafts. Anti-CD25 treatment was administered once, the day before second heart transplantation (n=6 each). Controls (post Lm, n=6) are the same as shown in Fig. 1d. Data are presented as percent graft survival of the second B/c hearts with or without anti-CD25 therapy (P<0.01 by log-rank test) and are pooled from three experiments.