Table 2.
Intermolecular gold(I)-catalyzed [2+2] cycloaddition between alkynes (1a–n) and α-methylstyrene (2a)[a]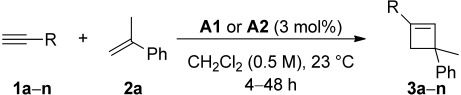
| Entry | R | Catalyst | Product (Yield [%])[b] |
|---|---|---|---|
| 1 | Ph (1a) | A1 | 3a (80)[c] |
| 2 | A2 | 3a (95) | |
| 3 | p-Tol (1b) | A1 | 3b (74)[c] |
| 4 | A2 | 3b (86) | |
| 5 | p-MeOC6H4 (1c) | A1 | 3c (68)[c] |
| 6 | A2 | 3c (64) | |
| 7 | p-FC6H4 (1d) | A1 | 3d (75)[c] |
| 8 | A2 | 3 d (84) | |
| 9 | p-ClC6H4 (1e) | A1 | 3e (61)[c] |
| 10 | A2 | 3 e (91) | |
| 11 | p-BrC6H4 (1f) | A1 | 3f (74)[c] |
| 12 | A2 | 3 f (97) | |
| 13 | m-MeOC6H4 (1g) | A1 | 3g (80) |
| 14 | A2 | 3g (78) | |
| 15 | m-Tol (1h) | A1 | 3h (78)[c] |
| 16 | A2 | 3h (91) | |
| 17 | m-HOC6H4 (1i) | A1 | 3i (74)[c] |
| 18 | A2 | 3i (98) | |
| 19 | m-FC6H4 (1j) | A1 | 3j (67) |
| 20 | A2 | 3j (77) | |
| 21 | m-ClC6H4 (1k) | A1 | 3k (60) |
| 22 | A2 | 3k (83) | |
| 23 | o-MeOC6H4 (1l) | A1 | 3l (30) |
| 24 | A2 | 3l (24) | |
| 25 | 3-thienyl (1m) | A1 | 3m (84) |
| 26 | A2 | 3m (86) | |
| 27 | cyclopropyl (1n) | A1 | 3n (46)[c] |
| 28 | A2 | 3n (35) |
