Figure 1. CONSORT diagram for the Costa Rica Vaccine Trial and PATRICIA trial combined.
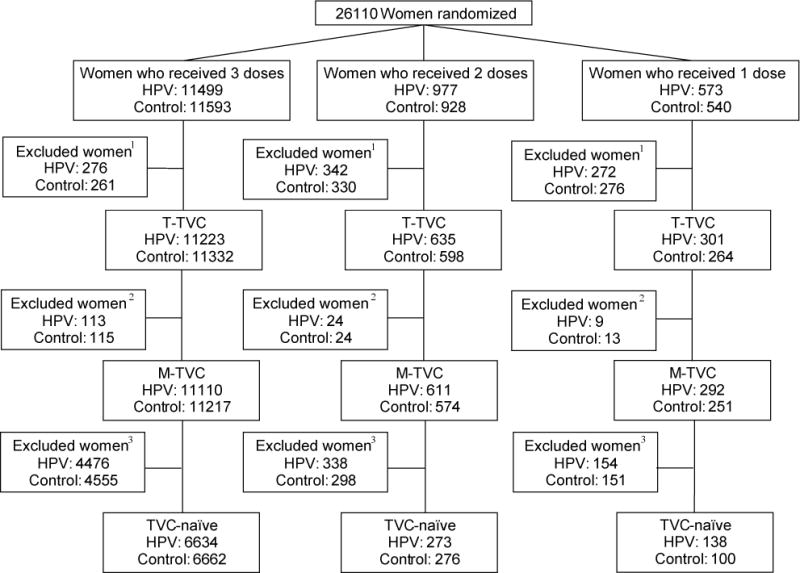
This CONSORT contains women who were randomized into both trials, stratified by vaccine arm and number of doses received. 1Women were excluded if they had less than 12 months follow-up time (time total vaccinated cohort, T-TVC). 2Women were additionally excluded if their enrollment cervical status was HPV16 AND 18 DNA-positive (or missing) or they had fewer than 300 days between first and last PCR result; modified total vaccinated cohort (M-TVC) evaluating HPV16/18-related endpoints. 3Women were further excluded from the TVC-naïve if their cervical status at enrollment was HPV DNA-positive for any oncogenic type (or missing), or HPV16 or 18 seropositive (or missing), or had enrollment cytology abnormal (or missing).
