Fig. 3.
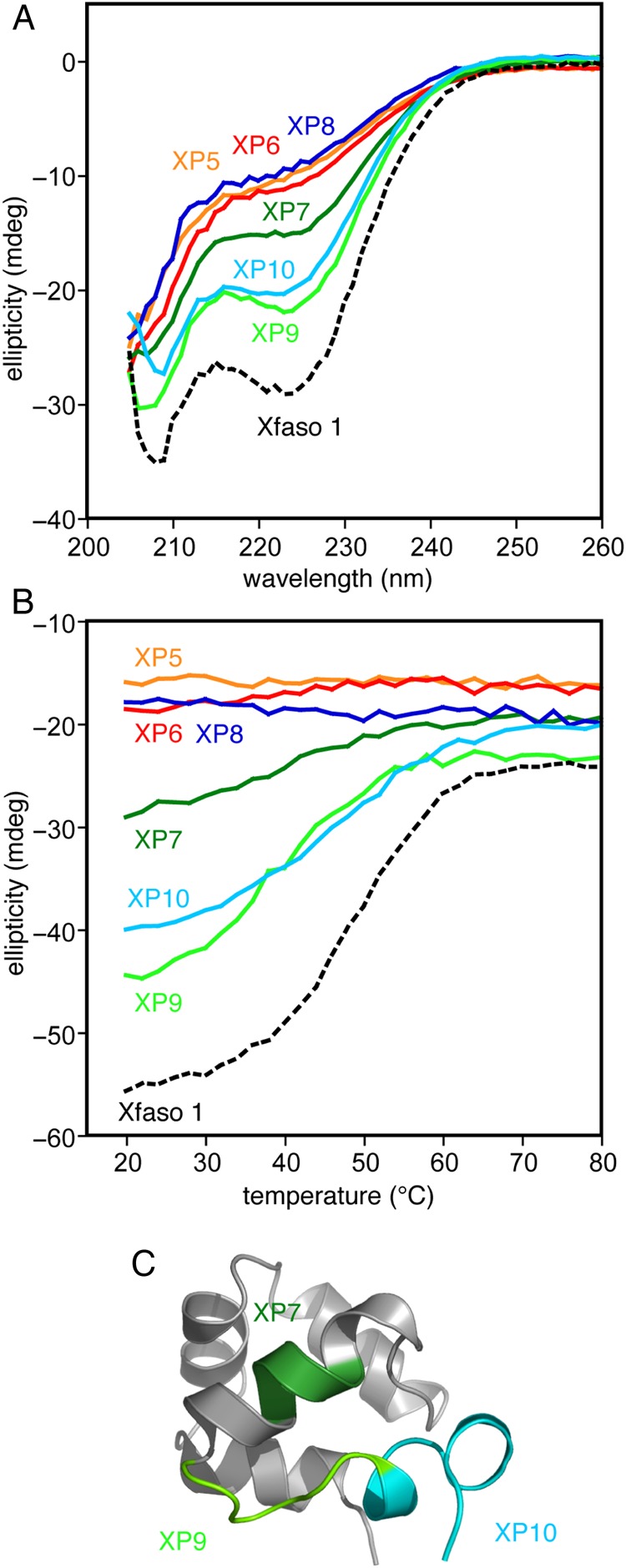
Block substitutions of Pfl 6 C-terminal sequence into Xfaso 1 show varying abilities to fold: (A) far ultraviolet CD spectra (25 μM protein at 1 mm pathlength, 20°C) of eight block hybrids compared with the parent sequence, wild-type Xfaso 1, (B) thermal denaturation of the block hybrids monitored by CD at 222 nm (25 μM protein at 1 mm pathlength, 20°C), (C) regions for which the block substitutions are reasonably well tolerated, mapped onto the subunit structure of Xfaso 1. See Table I for sequences.
