Figure 3.
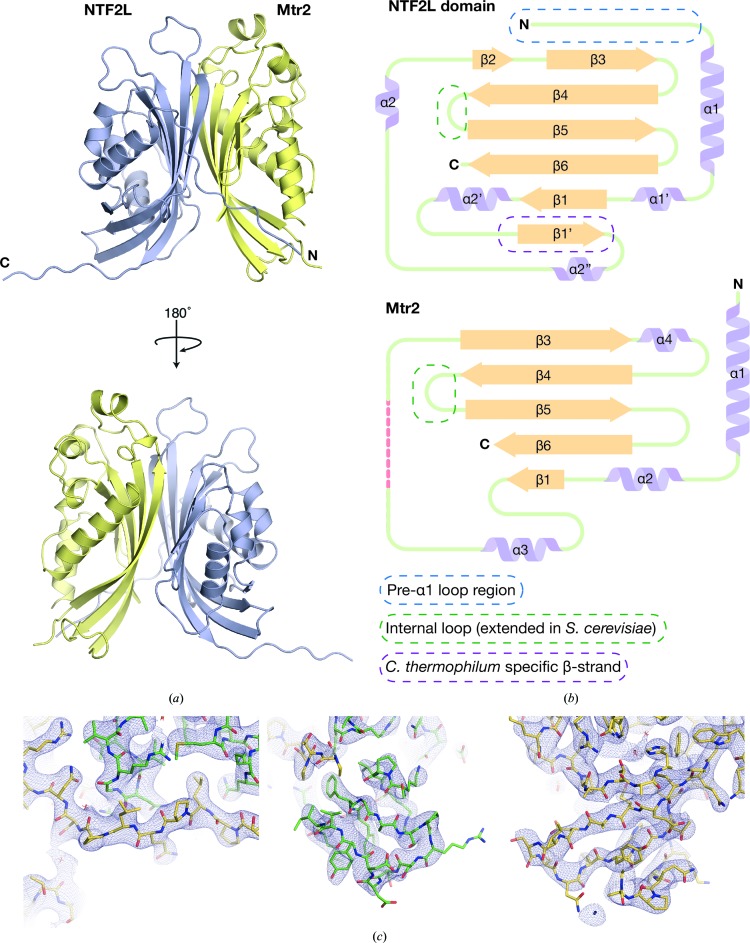
(a) Overview of the 2.9 Å resolution crystal structure of ctMex67NTF2L–Mtr2. The two chains are related in a twofold-symmetric manner, where the highly curved β-sheets form a tight heterodimeric complex. (b) Schematic illustration of the secondary-structural elements in the NTF2L domain and Mtr2. Disordered regions are shown as red dotted lines. The internal loop present between β4 and β5 in both the NTF2L domain and Mtr2 were ordered, but not extended as shown to be the case in S. cerevisiae (circled with a dotted green line). The pre-α1 loop region of the NTF2L domain was also ordered in ctMex67 and was bound across Mtr2 in an analogous way to that seen in hsNXF1NTF2L–NXT1 (PDB entry 1jkg; Fribourg et al., 2001 ▸). An extra β-strand was present in the NTF2L domain when compared with the hsNXF1 NTF2L domain and was probably owing to a lattice contact involving the extreme C-terminus of the NTF2L domain (denoted β1′ and circled with a dotted purple line). (c) Three representative views of the final 2F o − F c maps for the ctMex67NTF2L–Mtr2 structure contoured at the 1σ level (the ctMex67NTF2L domain is shown in yellow and ctMtr2 is shown in green).