Abstract
Reactive oxygen species regulate cardiovascular and renal function in health and disease. Superoxide participates in acute calcium signaling in afferent arterioles and renal vasoconstriction produced by angiotensin II, endothelin, thromboxane and pressure-induced myogenic tone. Known mechanisms by which superoxide acts include quenching of nitric oxide and increased ADP ribosyl cyclase/ryanodine-mediated calcium mobilization. The effect(s) of superoxide on other calcium signaling pathways in the renal microcirculation is poorly understood. The present experiments examined the acute effect of superoxide generated by paraquat on calcium entry pathways in isolated rat afferent arterioles. The peak increase in cytosolic calcium concentration caused by KCl (40 mM) was 99 ± 14 nM. The response to this membrane depolarization was mediated exclusively by L-type channels as it was abolished by nifedipine but was unaffected by the T-type channel blocker mibefradil. Paraquat increased superoxide production (dihydroethidium fluorescence), tripled the peak response to KCl to 314 ± 68 nM (p<0.001) and doubled the plateau response. These effects were abolished by tempol and nitroblue tetrazolium, but not by catalase, confirming actions of superoxide and not hydrogen peroxide. Unaffected by paraquat and superoxide was calcium entry through store-operated calcium channels activated by thapsigargin-induced calcium depletion of sarcoplasmic reticular stores. Also unresponsive to paraquat was ryanodine receptor-mediated calcium-induced calcium release from the sarcoplasmic reticulum. Our results provide new evidence that superoxide enhances calcium entry through L-type channels activated by membrane depolarization in rat cortical afferent arterioles, without affecting calcium entry through store operated entry or ryanodine receptor-mediated calcium mobilization.
Keywords: renal, nephrology, reactive oxygen species, superoxide, calcium signaling, calcium entry, hypertension
INTRODUCTION
Reactive oxygen species (ROS), such as superoxide and hydrogen peroxide (H2O2), influence vascular physiology and pathophysiology 1, 2. in the renal vasculature and tubules is an important negative modulator of nitric oxide (NO), a vasodilator and natriuretic agent, by limiting its availability. Together these two opposing radicals provide an important balance in regulating the magnitude of vasoconstriction, sodium excretion and blood pressure (BP)1. In addition to reducing NO bioavailability, acts directly on vascular smooth muscle cells (VSMC) to augment calcium (Ca2+) signaling and enhance vasoconstriction 1, 3, 4. Our laboratory has observed that participates in and amplifies acute renal vasoconstrictor responses induced by angiotensin II (Ang II) 5, endothelin-1 (ET-1) 6, and thromboxane (TxA2) 7 and stimulation of cytosolic calcium (Ca2+) in the afferent arteriole by these agents 8, 9. was implicated as the critical ROS, based on attenuation by dismutation of by tempol. Other investigators have reported that mediates the acute renal vasoconstriction produced by Ang II in normotensive and hypertensive animals with attenuation by antioxidants 5, 10-12. Moreover, potentiates the strength of the myogenic response of cortical afferent arterioles 13-15.
Excessive vascular and renal lead to vascular dysfunction and/or disturbed salt and water homeostasis 1, 16, 17. Oxidative stress caused by increased ROS levels and NO deficiency is associated with renal vasoconstriction and the development of Ang II-induced and salt-sensitive hypertension 1, 3, 18-21. activity is enhanced in NO deficient rats, and contributes to abnormal renal function 1, 22. For example, increased activity is responsible for inducing salt-sensitive hypertension in endothelial NO synthase knockout mice 19, 22. Administration of superoxide dismutase (SOD) effectively reduces BP in salt-sensitive and salt-independent models of hypertension 1, 23. Knockout mice deficient in extracellular SOD-3 have a higher basal BP than wild-type mice, a phenotype attributed to higher and decreased NO levels in the kidney 24. Moreover, chronic Ang II administration produces more pronounced hypertension in SOD-3 deficient mice than in wild-type controls 25.
Ang II-induced hypertension is coupled with oxidative stress in blood vessels 26, 27, and increased renal and non-renal vascular ROS is a common feature in both saltindependent and salt-sensitive hypertension 1, 20, 21, 28. A ROS-dependent rise in renal vascular resistance (RVR) and BP is observed in Ang II-infused hypertensive mice and rats 29, 30. Augmented oxidative stress in the spontaneously hypertensive rat (SHR) involves overexpression of NADPH oxidase and loss of extracellular SOD in the kidney 31. The SOD mimetic tempol normalizes elevated basal RVR and BP and restores endothelial function of renal arteries in the SHR and in the 2 kidney, 1 clip Goldblatt model of renovascular hypertension 32, 33, further implicating in exaggerated renal vasoconstriction and the potentiation of hypertension during oxidative stress 34. Therefore, increased intrarenal ROS or an abnormal balance can alter renal hemodynamics and sodium excretion to cause hypertension 1.
The precise mechanism(s) by which affects Ca2+ signaling and causes contraction of VSMC in the renal microcirculation is poorly understood. Ca2+ signaling studies have linked G-protein coupled receptors (GPCR) for Ang II, ET-1 and catecholamines, and TxA2 to rapid production and sensitization of RyR to mobilize Ca2+ from sarcoplasmic reticular stores in the renal vasculature 5, 7, 9, 35. In cerebral arteries, ROS generation by hypoxanthine/xanthine oxidase (HX/XO), and by Ang II stimulation activates L-type channels to promote Ca2+ entry from the extracellular fluid 36. Many GPCR ligands and perfusion pressure elicit contraction of afferent arterioles by stimulating Ca2+ entry through L-type channels 37. Interactions between and L-type Ca2+ channel activity in the renal microcirculation have not been investigated.
METHODS
See details in Methods in the online-only Data Supplement. All animal studies were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of North Carolina.
Preparation of afferent arterioles
Afferent arterioles (<20 μm in diameter) were isolated from 3 to 6 wk-old (50–120 g) male Sprague-Dawley rats using a magnetized iron oxide-sieving technique as previously described in our laboratory 8, 9. We elected to use young animals because the preparation and purification of single afferent arterioles with little advential tissue from young rats were technically much easier than from mature rats. Pilot studies established that the increase in cytosolic Ca2+ concentration produced by 40 mM KCl was similar in young and adult rat afferent arterioles (Online supplement, please see http://hyper.ahajournals.org). All animal research conducted adhered to the NIH Guide for the Care and Use of Laboratory Animals.
Measurement of paraquat-induced cytosolic production
We used paraquatto generate in fresh afferent arterioles. Paraquat (N,N’- dimethyl-4,4’-bipyridinium dichloride) is a classic and well established model for longterm oxidant-initiated toxicity due to its ability to generate . Paraquat redox cycles with cellular diaphorases and molecular oxygen to generate intracellular at levels that do not acutely affect cell viability 39, 40. We selected 1 mM paraquat for testing because it causes marked afferent arteriolar constriction that is reversed by tempol without adversely affecting short-term cell viability 35.
production was measured in isolated afferent arterioles using the oxidative-responsive fluorescent dye dihydroethidium (DHE) as previously described 7. After a baseline recording of a 90 sec incubation period with paraquat (1mM) or PBS alone as a negative control, a final image was acquired. Some arterioles were incubated with tempol (1 mM) or catalase (250 U/ml) in PBS for 20 min before testing the response to paraquat.
Measurement of cytosolic free calcium concentration
[Ca2+]i was measured in individual afferent arterioles as previously described 8, 41, 42. Fura-2 fluorescence was detected using a CCD camera (Digital Video Camera Co. DVC 1500) after passing through a 510 nm emission filter. [Ca2+]i was determined by ratiometric analysis of Fura-2 emission intensities at two excitation wavelengths (340 nm, 380 nm). Signal intensity was acquired and processed using InCytIm2 software (Intracellular Imaging, Cincinnati, OH).
Protocols
Values for each arteriole were averaged and analyzed to identify the peak and the plateau phases of the response. Peak values were the average of three data points corresponding to the highest measured [Ca2+]i after stimulation. Plateau responses were defined as the average of the 5 data points occurring 45 sec (43-47 sec) after the peak response.
To determine the relative contributions of L-type and T-type channels to Ca2+ entry resulting from KCl-induced membrane depolarization, we used selective L-type and Ttype Ca2+ channel inhibitors nifedipine (1 μM) and mibefradil (1 μM). Arterioles were incubated for 10 min in HBSS containing either channel blocker prior to stimulation with 40 mM KCl.
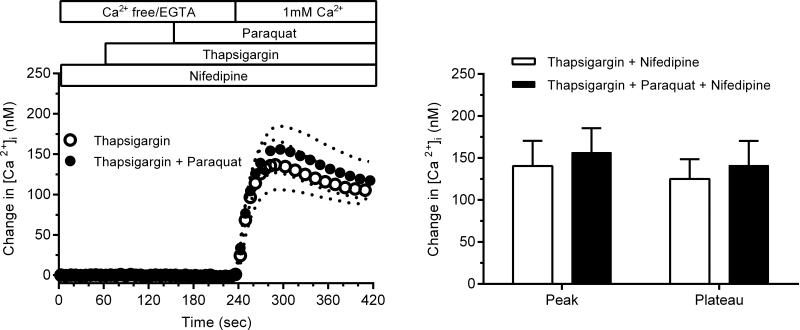
To test the effects of on Ca2+ entry through L-type channels, paraquat was added 90 sec prior to stimulation with 40 mM KCl. The effect of paraquat on Ca2+ entry via SOC was determined using a protocol previously described by our laboratory 43. Arterioles were incubated with Ca2+-free HBSS containing 10 μM thapsigargin for 20 min and then the thapsigargin-containing HBSS was replaced with a bathing medium composed of Ca2+-free PBS with 0.5 mM EGTA and 10 μM thapsigargin. Paraquat (1 mM) was added to the bathing medium 90 sec prior to switching the bath solution to PBS containing Ca2+ (1 mM).
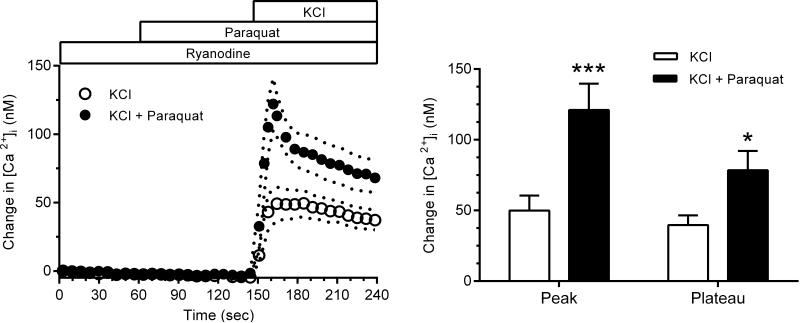
To assess the contribution from Ca2+-induced Ca2+ release (CICR), arterioles were incubated in HBSS containing 50 μM Ry for 20 min to block Ry receptors (RyR) and prevent CICR 41. After incubation, arterioles were placed in a bathing medium composed of PBS with 1.1 mM Ca2+ and 50 μM Ry. In other experiments, paraquat (1 mM) was added 90 sec prior to KCl stimulation.
Statistics
A two-tailed Student's t-test was used to determine significance between groups (Prism Software). P < 0.05 was determined to be significant.
RESULTS
The results for [Ca2+]i are presented as nM ± SEM change from baseline. Baseline [Ca2+]i for all afferent arterioles studied averaged 100 ± 4 nM (n=111). Basal values for each group of experiments did not differ statistically from the overall mean (p>0.1).
High KCl stimulates Ca2+ entry through L-type channels
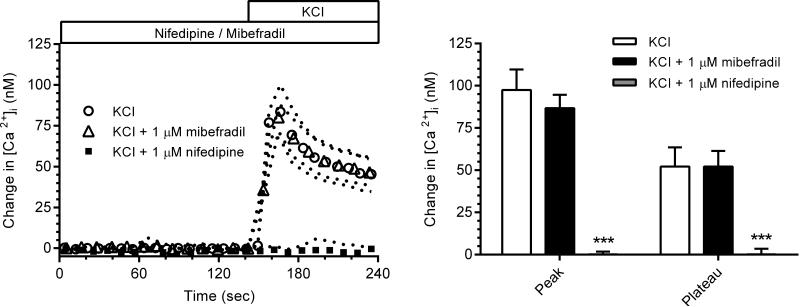
Fig. 1 (left panel) shows high KCl (40 mM)-induced depolarization of the plasma membrane of VSMC of afferent arterioles to stimulate Ca2+ influx to increase [Ca2+]i.. The initial peak increase in [Ca2+]i averaged 99 ± 14 nM while the sustained plateau phase at 45 s after addition of KCl was 53 ± 10 nM (Fig. 1, right panel). Fig. 1 (right and left panels) shows the complete abolition of high KCl-induced Ca2+ entry when L-type channels were blocked by nifedipine (1 μM). Addition of the T-type inhibitor mibefradil (1 μM) did not alter Ca2+ responses to high KCl. These observations support previous reports that KCl-induced depolarization exclusively activates voltage-gated L-type Ca2+ channels in the rat cortical afferent arteriole 44-47.
Fig. 1.
Left panel: [Ca2+]i responses to KCl in the presence of the L-type and T-type Ca2+ channel inhibitors, nifedipine (n=7) and mibefradil (n=8), respectively, are compared to a control KCl response (n=8). Right Panel: Effects of nifedipine and mibefradil on average peak and plateau [Ca2+]i responses. (*** p<0.005)
Paraquat incresees production in afferent arteriolar VSMCs
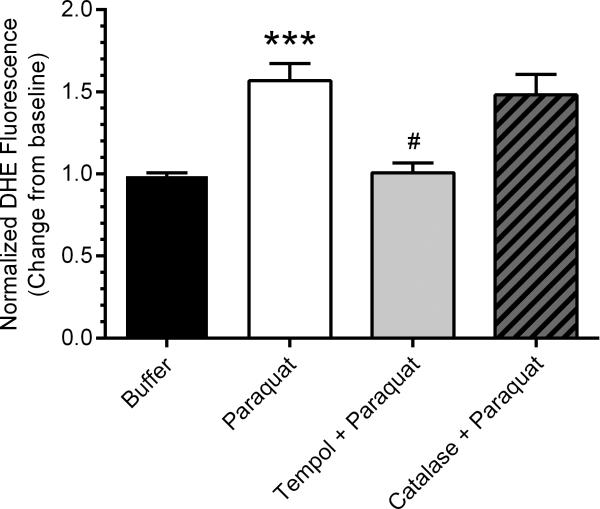
Fig. 2 shows that paraquat (1 mM) induced a 50% increase in generation (DHE fluorescence) in these afferent arteriolar VSMCs. This increase in was abolished by tempol (p<0.001) and not affected by catalase.
Fig. 2.
Paraquat elicited a 50% increase in cytosolic (DHE fluorescence, n=7, filled bar) as compared to buffer as a negative control (n=7, open bar) and paraquat (n=7, filled bar). The paraquat-induced increase in production was abolished by tempol (n=7, p<0.005, gray bar), but was not significantly influenced by catalase (n=7, patterned bar) (*** p<0.005 vs. control, # p<0.005 vs. paraquat).
Paraquat and generation increase Ca2+ influx induced by membrane depolarization
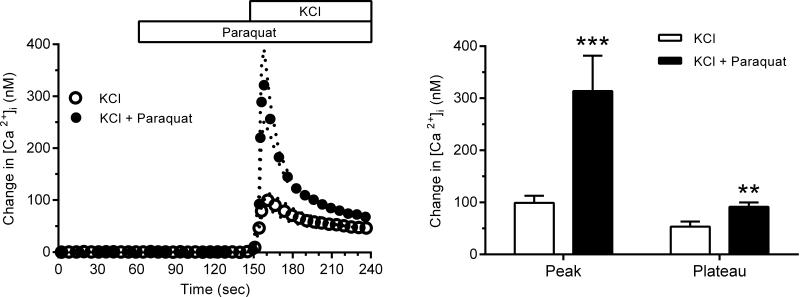
Fig. 3 (left panel) demonstrates that the addition of paraquat (10−3 M) to generate of markedly enhanced the Ca2+ entry through L-type Ca2+ channels stimulated by KCl-induced membrane depolarization but not during basal conditions before addition of KCl. Paraquat increased both the peak and the plateau phases of the response to KCl (Fig. 3, right panel). The peak was enhanced 3-fold to 314 ± 68 nM and the plateau phase was augmented nearly 2-fold to 92 ± 8 nM (p<0.01 for both).
Fig. 3.
Left panel: [Ca2+]i response of freshly isolated afferent arterioles to KCl-induced (40 mM) membrane depolarization before (n=14, open circles) and after paraquat stimulation (n=10, filled circles). Note that paraquat did not affect basal [Ca2+]i prior to stimulation with KCl. Right panel: Average peak and plateau (45 sec after the peak) responses to KCl in the presence and absence of paraquat (*** p<0.005, ** p<0.01).
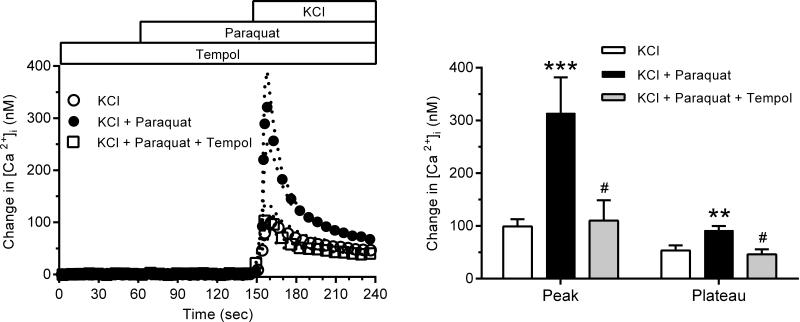
Fig. 4 (left panel) illustrates the effect of dismutation by tempol on the [Ca2+]i response to 40 mM KCl and paraquat stimulation. Tempol (10−3 M) had no effect on baseline [Ca2+]i before KCl stimulation, but abolished the enhancement produced by paraquat, yielding results approximating those of KCl alone in the absence of paraquat. Fig. 4 (right panel) shows that the effect of paraquat induced production to increase both peak and plateau Ca2+ responses to KCl was eliminated by the antioxidant tempol. Similar inhibition of the paraquat stimulation was observed when NBT was used to scavenge (NBT results online supplement only, please see http://hyper.ahajournals.org).
Fig. 4.
Left panel: The [Ca2+]i responses to KCl in the presence and absence of paraquat (closed and filled circles, respectively) (same as in Fig. 4), and the effect of tempol dismutation of paraquat-generated (n=8, open squares). Right panel: Effect of tempol on the average peak and plateau [Ca2+]i values. (*** p<0.005 vs. control, # p<0.05 vs. paraquat).
Paraquat and generation does not increase Ca2+ entry through SOC channels
To verify that Ca2+ entry took place through SOC and not L-type Ca2+ channels, vessels were treated with nifedipine throughout the experiment. Fig. 5 (left panel) displays the magnitude of Ca2+ entry through SOC in the presence and absence of generated by paraquat. Fig. 5 (right panel) shows the peak and plateau values for [Ca2+]i following activation of Ca2+ entry through SOC. Pretreatment with paraquat had no significant effect on Ca2+ entry through SOC during either phase (P>0.5).
Fig. 5.
Left panel: The magnitude of Ca2+ entry through SOC after thapsigargin-induced depletion of sarcoplasmic reticular Ca2+ stores and the lack of effect of paraquat (n=8 for both). Right panel: Peak and plateau values for [Ca2+]i as a result of Ca2+entry through SOC before and after addition of paraquat.
Enhancement of Ca2+ influx by paraquat does not involve CICR
Increased [Ca2+]i resulting from Ca2+ entry through L-type channels is known to increase CICR from the sarcoplasmic reticulum mediated by activation of the ryanodine receptor (RyR) 41. To test for a possible effect of on RyR-mediated CICR, we used Ry (50 μM) to inactivate RyR and prevent CICR secondary to Ca2+ entry stimulated by high KCl. As Fig. 6 (left panel) shows, during RyR inactivation to prevent CICR, paraquat enhanced the peak and plateau [Ca2+]i responses initiated by membrane depolarization (p<0.05), again demonstrating the ability of paraquat-derived to stimulate L-type channels. Also shown in Fig. 6, Ry decreased the [Ca2+]i response to high KCl, reducing the peak by 50% and the plateau phase by 23%. Thus, RyR-mediated CICR contributes to the [Ca2+]i response when Ca2+ entry is stimulated by membrane depolarization. Inactivation of RyR blunted the peak and plateau Ca2+ signals after paraquat to approximately the same extent (Fig. 6, right panel). enhanced KCl-induced Ca2+ influx during paraquat treatment, suggesting did not affect RyR-mediated Ca2+ mobilization in these experiments. Therefore, we conclude that the paraquat-induced increase in Ca2+ entry during membrane polarization is primarily due to L-type channel enhancement, and not by recruitment of SOC or augmented CICR.
Fig. 6.
Left panel: [Ca2+]i responses to KCl during inactivation of RyR-induced CICR in the presence (n=9) and absence (n=8) of paraquat. Right panel: Effect of paraquat on the average peak and plateau [Ca2+]i responses (*** p<0.005, * p<0.05).
DISCUSSION
This study evaluated the effects of generated by paraquat on Ca2+ signaling in VSMCs of rat renal afferent arterioles. was verified as the primary ROS responsible for increased Ca2+ influx based on DHE measurement and effective abolition of the paraquat effect on [Ca2+]i by either tempol or NBT (NBT results online supplement only, please see http://hyper.ahajournals.org). Moreover, production was negated by tempol but not catalase. Our major new finding is that enhanced Ca2+ entry in response to membrane depolarization induced by high extracellular KCl. We conclude that acted to increase Ca2+ entry by increasing the activity of L-type Ca2+ channels. The stimulation was rapid, requiring at most 90 sec of exposure to paraquat and was effective upon stimulation with KCl, whereas basal [Ca2+]i before stimulation was unaffected.
Our results extend previous studies showing that membrane depolarization by high KCl exclusively activates L-type Ca2+ channels to increase [Ca2+]i and contract the rat cortical afferent arteriole with little to no participation of T-type channels sensitive to mibefradil 44-46. Both responses were abolished by removal of extracellular Ca2+ or by pharmacological inhibition of voltage-gated L-type channels using nifedipine or nitrendipine 44-46. Based on patch clamp studies of voltage-activated Ca2+ currents, freshly isolated VSMCs of the rat afferent arteriole have a high density of L-type channels but do not express functionally active voltage-dependent T-type Ca2+ channels 47. In rat juxtamedullary afferent arterioles, it is reported that T-type channels are functionally expressed, but do not contribute to constrictor responses to KCl 48. On the other hand, another study reports that high KCl-induced Ca2+ entry is attenuated in rat juxtamedullary afferent arterioles and rabbit cortical afferent arterioles during inhibition of T-type channels by mibefradil 49.
Our results provide important insight into physiological and pathophysiological mechanisms within the kidney as L-type Ca2+ channel activity is a critical determinant of contractile tone of the afferent arteriole, whether stimulated by GPCR agonists or increased renal perfusion pressure 37. is known to modulate renal hemodynamics 1 and vasoconstrictor responses to GPCR agonists such as Ang II, ET-1 and PE 5, 6. Our finding of a direct action of on Ca2+ entry provides a mechanism to explain our previous demonstration that NADPH-derived mediates the afferent arteriolar [Ca2+]i response to Ang II and ET-1 8, 9. The immediate increase in [Ca2+]i stimulated by either agonist was attenuated by both tempol and apocynin, indicating participation of NADPH-derived . Moreover, the actions of Ang II and ET-1 were attenuated by 8-Br-cyclic ADPR and nicotinamide, implicating involvement of ADPR cyclase and RyR in the Ca2+ signaling pathway, steps proposed to be downstream of production.
ADPR cyclase synthesis of cADPR can sensitize RyR to [Ca2+]i to enhance CICR 50. Our earlier studies of afferent arterioles showed that RyR-mediated CICR also contributes to the increased [Ca2+]i following membrane depolarization induced by high KCl 41. Locking the RyR in the closed position with a high concentration of ryanodine (50-100 μM) attenuated ~50% of the [Ca2+]i response to Ca2+ entry through voltagegated L-type channels. Our current studies confirm this finding and extend it in that generated by paraquat had essentially no effect on the RyR-mediated CICR response to increased [Ca2+]i secondary to KCl-induced depolarization.
In our studies of freshly isolated afferent arterioles, paraquat did not elicit a change in basal [Ca2+]i, suggesting no acute effect of on Ca2+ entry in the absence of KCl-induced membrane depolarization when L-type channels are quiescent. This contrasts with other published reports that ROS increased basal [Ca2+]i in VSMC from nonrenal vessels 36, 51. Differences among studies may stem from ROS exposure times and concentrations, intracellular vs extracellular ROS generation, and vascular beds. It is noteworthy that HX/XO primarily generates extracellular ROS, whereas paraquat stimulates intracellular production. Our 90 sec exposure time to paraquat / was considerably shorter than some previous studies. In cultured rat mesenteric arterial VSMCs, ROS generated by cell membrane permeable LY23583 increased Tempo-9AC florescence after 25 min, a response abolished by tempol, whereas [Ca2+]i was increased after 15 min 50. In these nonrenal VSMC of WKY but not SHR, the increase was attributed to augmented Ca2+ influx through both L-type and T-type Ca2+ channels as it was inhibited by putative selective antagonists verapamil/diltiazem (10 μM) and mibefradil (10 μM), respectively 51.
Amberg et al. investigated the role of ROS in activating Ca2+ entry via L-type Cav1.2 Ca2+ channels and the resultant constriction of pressurized, freshly isolated cerebral arteries 36. Total internal reflection fluorescence microscopy revealed that Ang II and endogenous ROS rapidly stimulated Ca2+ entry by increasing L-type Ca2+ channel sparklet activity. Both the Ang II-induced increase in Ca2+ sparklets and arterial tone were abolished by apocynin inhibition of NADPH oxidase, implicating a stimulatory role of ROS, either or H2O2. Additionally, exogenous ROS generated by HX/XO increased PKCα and L-type Ca2+ channel activity and cerebral arterial vasomotor tone as a result of Ca2+ entry within 2 min of addition; the latter was abolished by inhibition of L-type channel activity with diltiazem 36. Exogenous ROS increased both Ca2+ sparklet activity and sparklet site density in voltage clamped arterial myocytes, further supporting the conclusion that ROS increases Ca2+ influx through L-type channels.
L-type channels are sensitive to oxidants, potentially due to direct redox modification of cysteines on the channel, or by redox modification of regulatory proteins involved in channel function 52-54. may amplify Ca2+ entry through L-type Ca2+ channels by promoting clustering that leads to cooperative gating 55. In this manner, increased production during ANG II-induced hypertension may explain increased L-type Ca2+ channel sparklet activity in arterial VSMC 56, 57 and the increased density of L-type channels 58. Another new finding of our present studies is that does not influence Ca2+ entry mediated by SOC in freshly isolated afferent arterioles. In cultured VSMC of porcine coronary artery and bovine pulmonary artery, ROS generated by HX/XO was reported to stimulate [Ca2+]i by inhibiting plasma membrane Ca2+-ATPase and SERCA 59-62. Such inhibition is predicted to deplete intracellular Ca2+ stores and thereby indirectly enhance SOC entry. Both TRPC and STIM-Orai channels have been proposed to be the primary SOC channels in other vascular beds 63, 64. AVP stimulation of and H2O2 production is reported to increase TRPC6 channel activity and Ca2+ influx in the A7r5 line of cultured VSMC and cultured mouse aortic VSMC 65. Presently, the molecular identity of SOC channels in the renal microcirculation is unknown.
In summary, our major novel finding is that enhances Ca2+ entry through L-type channels in VSMCs of freshly isolated afferent arterioles. We found no evidence for potentiation by paraquat of either Ca2+ entry through SOC or Ca2+ mobilization and CICR mediated by RyR.
PERSPECTIVES
It is well established that ROS play a pathophysiological role in the development of hypertension, however the specific mechanisms by which ROS alter renal hemodynamics in health and disease are poorly understood. interacts with NO and participates in rapid, acute constriction of the afferent arteriole and increased renal vascular resistance, but the effects on Ca2+ signaling pathways in the renal microcirculation are not known. Our studies provide new information that acts to enhance Ca2+ influx through L-type Ca2+ channels in the afferent arteriole, the major preglomerular resistance vessel in the kidney where Ca2+ entry through voltage-gated channels is a predominant Ca2+ signaling pathway. This stimulatory effect can be reversed both by dismutation with tempol and by scavenging with NBT. Ca2+ entry through store-operated channels resulting from thapsigargin-induced intracellular Ca2+ depletion of SR stores is not markedly influenced by cellular levels.
During renal autoregulation, changes in tone of the cortical radial arteries and afferent arterioles result from pressure-induced activation of L-type Ca2+ channels to maintain RBF, glomerular filtration rate, and buffer pressure-natriuresis. Acute increases in modulate the efficiency of renal autoregulation by augmenting the myogenic response of afferent arterioles 13, 15. An enhancement in L-type channel activity by may provide a mechanistic explanation for these observations. Increased production participates in renal vasoconstriction and sodium retention during the development of hypertension 1, 10, 11, 34, 66. The ability of to increase Ca2+ entry through L-type channels is likely to play an important role in the vasoconstriction of the preglomerular vasculature often associated with sodium retention and the development and maintenance of hypertension.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
Our studies provide new information that acts to enhance Ca2+ influx through L-type Ca2+ channels in the afferent arteriole, the major preglomerular resistance vessel in the kidney.
We also show that superoxide does not participate in other Ca2+ signaling mechanisms we investigated, such as store operated calcium entry and ryanodine receptor-mediated calcium mobilization and subsequent calcium-induced-calcium-release.
What is relevant?
The ability of to increase Ca2+ entry through L-type channels is likely to play an important role in vasoconstriction the preglomerular vasculature often associated with sodium retention and the development of hypertension.
Summary
Our studies address a lack of understanding of the mechanisms by which superoxide affects Ca2+ signaling and causes contraction of VSMC of renal resistance arterioles.
Acknowledgments
SOURCE OF FUNDING
This research was supported by NIH research grant HL-02334.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Araujo M, Wilcox CS. Oxidative stress in hypertension: Role of the kidney. Antioxidants & redox signaling. 2014;20:74–101. doi: 10.1089/ars.2013.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Touyz RM, Briones AM. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertension research : official journal of the Japanese Society of Hypertension. 2011;34:5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 3.Schnackenberg CG. Physiological and pathophysiological roles of oxygen radicals in the renal microvasculature. American journal of physiology. Regulatory, integrative and comparative physiology. 2002;282:R335–R342. doi: 10.1152/ajpregu.00605.2001. [DOI] [PubMed] [Google Scholar]
- 4.Lob HE, Vinh A, Li L, Blinder Y, Offermanns S, Harrison DG. Role of vascular extracellular superoxide dismutase in hypertension. Hypertension. 2011;58:232–239. doi: 10.1161/HYPERTENSIONAHA.111.172718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Just A, Olson AJ, Whitten CL, Arendshorst WJ. Superoxide mediates acute renal vasoconstriction produced by Angiotensin II and catecholamines by a mechanism independent of nitric oxide. American journal of physiology. Heart and circulatory physiology. 2007;292:H83–H92. doi: 10.1152/ajpheart.00715.2006. [DOI] [PubMed] [Google Scholar]
- 6.Just A, Whitten CL, Arendshorst WJ. Reactive oxygen species participate in acute renal vasoconstrictor responses induced by ETA and ETB receptors. American journal of physiology. Renal physiology. 2008;294:F719–F728. doi: 10.1152/ajprenal.00506.2007. [DOI] [PubMed] [Google Scholar]
- 7.Moss NG, Vogel PA, Kopple TE, Arendshorst WJ. Thromboxane-induced renal vasoconstriction is mediated by the adp-ribosyl cyclase CD38 and superoxide anion. American journal of physiology. Renal physiology. 2013;305:F830–F838. doi: 10.1152/ajprenal.00048.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellner SK, Arendshorst W. Endothelin-a and -b receptors, superoxide, and Ca2+ signaling in afferent arterioles. American journal of physiology. Renal physiology. 2007;292:F175–F184. doi: 10.1152/ajprenal.00050.2006. [DOI] [PubMed] [Google Scholar]
- 9.Fellner SK, Arendshorst WJ. Angiotensin II, reactive oxygen species, and Ca2+ signaling in afferent arterioles. American journal of physiology. Renal physiology. 2005;289:F1012–F1019. doi: 10.1152/ajprenal.00144.2005. [DOI] [PubMed] [Google Scholar]
- 10.de Richelieu LT, Sorensen CM, Holstein-Rathlou NH, Salomonsson M. NO-independent mechanism mediates tempol-induced renal vasodilation in SHR. American journal of physiology. Renal physiology. 2005;289:F1227–F1234. doi: 10.1152/ajprenal.00116.2005. [DOI] [PubMed] [Google Scholar]
- 11.Feng MG, Dukacz SA, Kline RL. Selective effect of tempol on renal medullary hemodynamics in spontaneously hypertensive rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2001;281:R1420–R1425. doi: 10.1152/ajpregu.2001.281.5.R1420. [DOI] [PubMed] [Google Scholar]
- 12.Majid DS, Nishiyama A, Jackson KE, Castillo A. Superoxide scavenging attenuates renal responses to Ang II during nitric oxide synthase inhibition in anesthetized dogs. American journal of physiology. Renal physiology. 2005;288:F412–F419. doi: 10.1152/ajprenal.00294.2004. [DOI] [PubMed] [Google Scholar]
- 13.Lai EY, Wellstein A, Welch WJ, Wilcox CS. Superoxide modulates myogenic contractions of mouse afferent arterioles. Hypertension. 2011;58:650–656. doi: 10.1161/HYPERTENSIONAHA.111.170472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozawa Y, Hayashi K, Wakino S, Kanda T, Homma K, Takamatsu I, Tatematsu S, Yoshioka K, Saruta T. Free radical activity depends on underlying vasoconstrictors in renal microcirculation. Clinical and Experimental Hypertension. 2004;26:219–229. doi: 10.1081/ceh-120030231. [DOI] [PubMed] [Google Scholar]
- 15.Ren Y, D'Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. American journal of physiology. Heart and circulatory physiology. 2010;298:H1769–H1775. doi: 10.1152/ajpheart.00537.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison DG, Gongora MC, Guzik TJ, Widder J. Oxidative stress and hypertension. Journal of the American Society of Hypertension : JASH. 2007;1:30–44. doi: 10.1016/j.jash.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Montezano AC, Touyz RM. Oxidative stress, NOXs, and hypertension: Experimental evidence and clinical controversies. Annals of medicine. 2012;44(Suppl 1):S2–16. doi: 10.3109/07853890.2011.653393. [DOI] [PubMed] [Google Scholar]
- 18.Schulman IH, Zhou MS, Raij L. Nitric oxide, Angiotensin II, and reactive oxygen species in hypertension and atherogenesis. Current hypertension reports. 2005;7:61–67. doi: 10.1007/s11906-005-0056-6. [DOI] [PubMed] [Google Scholar]
- 19.Kopkan L, Majid DS. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension. 2005;46:1026–1031. doi: 10.1161/01.HYP.0000174989.39003.58. [DOI] [PubMed] [Google Scholar]
- 20.Manning RD, Jr., Meng S, Tian N. Renal and vascular oxidative stress and salt-sensitivity of arterial pressure. Acta physiologica Scandinavica. 2003;179:243–250. doi: 10.1046/j.0001-6772.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox CS. Reactive oxygen species: Roles in blood pressure and kidney function. Current hypertension reports. 2002;4:160–166. doi: 10.1007/s11906-002-0041-2. [DOI] [PubMed] [Google Scholar]
- 22.Kopkan L, Majid DSA. Enhanced superoxide activity modulates renal function in NO-deficient hypertensive rats. Hypertension. 2006;47:568–572. doi: 10.1161/01.HYP.0000200027.34925.93. [DOI] [PubMed] [Google Scholar]
- 23.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in Angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 24.Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill PS, Aslam S, Wang X, Ji H, Sandberg K, Jose P, Wilcox CS. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension. 2006;48:934–941. doi: 10.1161/01.HYP.0000242928.57344.92. [DOI] [PubMed] [Google Scholar]
- 25.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–481. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 26.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. The Journal of clinical investigation. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. The Journal of clinical investigation. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. Journal of the American Society of Nephrology : JASN. 2003;14:2775–2782. doi: 10.1097/01.asn.0000092145.90389.65. [DOI] [PubMed] [Google Scholar]
- 29.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of Angiotensin II slow pressor response: Role of oxidative stress. Journal of the American Society of Nephrology : JASN. 2002;13:2860–2868. doi: 10.1097/01.asn.0000035087.11758.ed. [DOI] [PubMed] [Google Scholar]
- 30.Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: Role of oxidative stress. American journal of physiology. Heart and circulatory physiology. 2005;288:H22–H28. doi: 10.1152/ajpheart.00626.2004. [DOI] [PubMed] [Google Scholar]
- 31.Adler S, Huang H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. American journal of physiology. Renal physiology. 2004;287:F907–F913. doi: 10.1152/ajprenal.00060.2004. [DOI] [PubMed] [Google Scholar]
- 32.Welch WJ, Mendonca M, Aslam S, Wilcox CS. Roles of oxidative stress and AT1 receptors in renal hemodynamics and oxygenation in the postclipped 2k,1c kidney. Hypertension. 2003;41:692–696. doi: 10.1161/01.HYP.0000052945.84627.8F. [DOI] [PubMed] [Google Scholar]
- 33.Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-iso prostaglandin F-2 alpha. Hypertension. 1999;33:424–428. doi: 10.1161/01.hyp.33.1.424. [DOI] [PubMed] [Google Scholar]
- 34.Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: Role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- 35.Schnackenberg CG, Welch WJ, Wilcox CS. TP receptor-mediated vasoconstriction in microperfused afferent arterioles: Roles of O(2)(−) and NO. American journal of physiology. Renal physiology. 2000;279:F302–F308. doi: 10.1152/ajprenal.2000.279.2.F302. [DOI] [PubMed] [Google Scholar]
- 36.Amberg GC, Earley S, Glapa SA. Local regulation of arterial L-type calcium channels by reactive oxygen species. Circulation research. 2010;107:U1002–U1123. doi: 10.1161/CIRCRESAHA.110.217018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navar LG, Arendshorst WJ, Pallone TL, Inscho EW, Imig JD, Bell PD. The renal microcirculation. Handbook of Physiology: Microcirculation. (2nd Edition.) 2008:550–683. [Google Scholar]
- 38.Bus JS, Gibson JE. Paraquat: Model for oxidant-initiated toxicity. Environmental Health Perspectives. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ody C, Junod AF. Direct toxic effects of paraquat and oxygen on cultured endothelial cells. Laboratory investigation; a journal of technical methods and pathology. 1985;52:77–84. [PubMed] [Google Scholar]
- 40.Gobbel GT, Chan TY, Chan PH. Nitric oxide- and superoxide-mediated toxicity in cerebral endothelial cells. The Journal of pharmacology and experimental therapeutics. 1997;282:1600–1607. [PubMed] [Google Scholar]
- 41.Fellner SK, Arendshorst WJ. Voltage-gated Ca2+ entry and ryanodine receptor Ca2+-induced Ca2+ release in preglomerular arterioles. American journal of physiology. Renal physiology. 2007;292:F1568–1572. doi: 10.1152/ajprenal.00459.2006. [DOI] [PubMed] [Google Scholar]
- 42.Fellner SK, Arendshorst WJ. Angiotensin II Ca2+ signaling in rat afferent arterioles: Stimulation of cyclic ADP ribose and IP3 pathways. American journal of physiology. Renal physiology. 2005;288:F785–F791. doi: 10.1152/ajprenal.00372.2004. [DOI] [PubMed] [Google Scholar]
- 43.Fellner SK, Arendshorst WJ. Store-operated Ca2+ entry is exaggerated in fresh preglomerular vascular smooth muscle cells of SHR. Kidney international. 2002;61:2132–2141. doi: 10.1046/j.1523-1755.2002.00383.x. [DOI] [PubMed] [Google Scholar]
- 44.Carmines PK, Fowler BC, Bell PD. Segmentally distinct effects of depolarization on intracellular [Ca2+] in renal arterioles. American journal of physiology. Renal physiology. 1993;265:F677–F685. doi: 10.1152/ajprenal.1993.265.5.F677. [DOI] [PubMed] [Google Scholar]
- 45.Loutzenhiser R, Epstein M. Activation mechanisms of human renal artery: Effects of KCl, norepinephrine and nitrendipine upon tension development and 45Ca influx. European journal of pharmacology. 1984;106:47–52. doi: 10.1016/0014-2999(84)90676-9. [DOI] [PubMed] [Google Scholar]
- 46.Loutzenhiser R, Hayashi K, Epstein M. Divergent effects of KCl-induced depolarization on afferent and efferent arterioles. American journal of physiology. Renal physiology. 1989;257:F561–F564. doi: 10.1152/ajprenal.1989.257.4.F561. [DOI] [PubMed] [Google Scholar]
- 47.Smirnov SV, Loutzenhiser K, Loutzenhiser R. Voltage-activated Ca(2+) channels in rat renal afferent and efferent myocytes: No evidence for the T-type Ca(2+) current. Cardiovascular research. 2013;97:293–301. doi: 10.1093/cvr/cvs310. [DOI] [PubMed] [Google Scholar]
- 48.Feng MG, Li M, Navar LG. T-type calcium channels in the regulation of afferent and efferent arterioles in rats. American journal of physiology. Renal physiology. 2004;286:F331–337. doi: 10.1152/ajprenal.00251.2003. [DOI] [PubMed] [Google Scholar]
- 49.Hansen PB, Jensen BL, Andreasen D, Skott O. Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circulation research. 2001;89:630–638. doi: 10.1161/hh1901.097126. [DOI] [PubMed] [Google Scholar]
- 50.Arendshorst WJ, Thai TL. Regulation of the renal microcirculation by ryanodine receptors and calcium-induced calcium release. Current opinion in nephrology and hypertension. 2009;18:40–49. doi: 10.1097/MNH.0b013e32831cf5bd. [DOI] [PubMed] [Google Scholar]
- 51.Tabet F, Savoia C, Schiffrin EL, Touyz RM. Differential calcium regulation by hydrogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. Journal of cardiovascular pharmacology. 2004;44:200–208. doi: 10.1097/00005344-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Hool LC. Evidence for the regulation of L-type Ca2+ channels in the heart by reactive oxygen species: Mechanism for mediating pathology. Clinical and experimental pharmacology & physiology. 2008;35:229–234. doi: 10.1111/j.1440-1681.2007.04727.x. [DOI] [PubMed] [Google Scholar]
- 53.Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and s-nitrosothiols. The Journal of general physiology. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupte SA, Wolin MS. Oxidant and redox signaling in vascular oxygen sensing: Implications for systemic and pulmonary hypertension. Antioxidants & redox signaling. 2008;10:1137–1152. doi: 10.1089/ars.2007.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Navedo MF, Amberg GC. Local regulation of L-type Ca2+ channel sparklets in arterial smooth muscle. Microcirculation. 2013;20:290–298. doi: 10.1111/micc.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and Angiotensin II-induced hypertension. Circulation research. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 57.Gulia J, Navedo MF, Gui PC, Chao JT, Mercado JL, Santana LF, Davis MJ. Regulation of L-type calcium channel sparklet activity by c-Src and PKC-alpha. American Journal of Physiology. Cell Physiology. 2013;305:C568–C577. doi: 10.1152/ajpcell.00381.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pesic A, Madden JA, Pesic M, Rusch NJ. High blood pressure upregulates arterial L-type Ca2+ channels: Is membrane depolarization the signal? Circulation research. 2004;94:e97–e104. doi: 10.1161/01.RES.0000131495.93500.3c. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki YJ, Ford GD. Inhibition of Ca(2+)-ATPase of vascular smooth muscle sarcoplasmic reticulum by reactive oxygen intermediates. American journal of physiology. Heart and circulatory physiology. 1991;261:H568–H574. doi: 10.1152/ajpheart.1991.261.2.H568. [DOI] [PubMed] [Google Scholar]
- 60.Grover AK, Samson SE, Fomin VP, Werstiuk ES. Effects of peroxide and superoxide on coronary artery: Ang II response and sarcoplasmic reticulum Ca2+ pump. American Journal of Physiology. Cell Physiology. 1995;269:C546–C553. doi: 10.1152/ajpcell.1995.269.3.C546. [DOI] [PubMed] [Google Scholar]
- 61.Grover AK, Kwan CY, Samson SE. Effects of peroxynitrite on sarco/endoplasmic reticulum Ca2+ pump isoforms SERCA2b and SERCA3a. American journal of physiology. Cell physiology. 2003;285:C1537–C1543. doi: 10.1152/ajpcell.00299.2003. [DOI] [PubMed] [Google Scholar]
- 62.Lounsbury KM, Hu Q, Ziegelstein RC. Calcium signaling and oxidant stress in the vasculature. Free Radical Biology and Medicine. 2000;28:1362–1369. doi: 10.1016/s0891-5849(00)00222-7. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Deng X, Gill DL. Calcium signaling by STIM and Orai: Intimate coupling details revealed. Science Signaling. 2010;3:pe42. doi: 10.1126/scisignal.3148pe42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW. Jr. Emerging perspectives in store-operated Ca2+ entry: Roles of Orai, STIM and TRP. Biochimica et biophysica acta. 2006;1763:1147–1160. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 65.Ding Y, Winters A, Ding M, Graham S, Akopova I, Muallem S, Wang Y, Hong JH, Gryczynski Z, Yang SH, Birnbaumer L, Ma R. Reactive oxygen species-mediated TRPC6 protein activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. The Journal of biological chemistry. 2011;286:31799–31809. doi: 10.1074/jbc.M111.248344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ichihara A, Hayashi M, Hirota N, Saruta T. Superoxide inhibits neuronal nitric oxide synthase influences on afferent arterioles in spontaneously hypertensive rats. Hypertension. 2001;37:630–634. doi: 10.1161/01.hyp.37.2.630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.