Fig. 1.
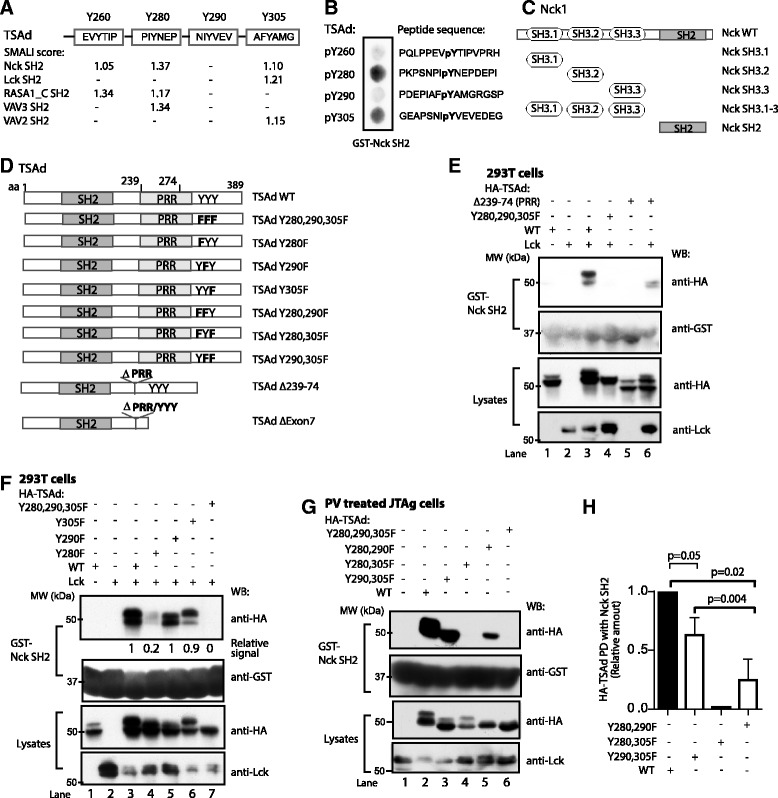
TSAd-pTyr280 and -pTyr305 interact with the Nck SH2 domain. a SH2 domain interaction partners to TSAd phosphotyrosines predicted by SMALI. The five best candidates are listed. Scores > 1 indicate a potential interaction.”-“indicates a SMALI score < 1. b Peptide spot array spotted with indicated TSAd phosphopeptides probed with GST-Nck SH2 and developed with anti-GST antibody. SH2: Src homology 2 domain, Y: tyrosine phosphorylation site. c and d Overview of the Nck (c) and TSAd (d) Constructs used. WT: wild type, SH3: Src homology 3 domain, PRR: proline rich region, Y: tyrosine phosphorylation site and F: phenylalanine. e-g Proteins pulled down by GST-Nck SH2 were resolved on SDS-PAGE, and immunoblotted with the indicated antibodies. e GST-Nck SH2 pull-down from 293T cells transiently transfected with Lck and the indicated HA-tagged TSAd cDNA constructs. Lck immunblot of the GST-Nck SH2 pulldown was negative (data not shown). f As in (e), including TSAd encoding single Y → F mutations. Relative TSAd binding to GST-Nck SH2 domain was analysed by ImageJ. Amount of pulled down HA-TSAd WT was set to 1. g GST-Nck SH2 pull-down from pervanadate treated JTAg cells transiently expressing indicated TSAd Y → F double mutations. Control GST pull-downs were negative (data not shown). Data shown are representative of at least three experiments (triple Y → F TSAd mutant and double mutants) or two experiments (Y → F single mutants). h Graph shows relative amounts of HA-TSAd that were pulled down (PD) with GST-Nck SH2 as in (g) measured by ImageJ. Amount of pulled down HA-TSAd WT was set to 1. Mean values ± SD of three independent experiments (2-tailed paired t-test)
