Table 1.
Reaction of zinc reagents with benzophenone in a hexane solution for 18 h. 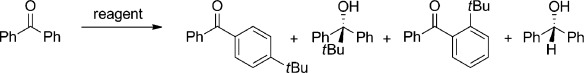
| Entry | Reagent | T | Yield [%][a] | |||||
|---|---|---|---|---|---|---|---|---|
| [°C] | para addi- tion (1,6) | Carbonyl addition (1,2) | ortho addi- tion (1,4) | benzhydrol (H− addition) | total | |||
| Stoichiometric | ||||||||
| 1 | tBu2Zn (1 equiv) | RT | 1 | 0 | 0 | 0 | 1 | |
| 2 | tBu2Zn⋅2 pyridine (1 equiv) | RT | 1 | 0 | 0 | 0 | 1 | |
| 3 | tBu2Zn⋅TMEDA (1 equiv) | RT | 1 | 1 | 0 | 0 | 2 | |
| 4 | [(TMEDA)Na(TMP)(tBu)Zn(tBu)] (1; 1 equiv) | RT | 58 | 14 | 3 | 0 | 75 | |
| 5 | [(TMEDA)2Na2(μ-dpa)2Zn(tBu)2] (3; 1 equiv) | RT | 40 | 6 | 0 | 11 | 57 | |
| 6 | [[Na(THF)6]+ [Zn(tBu)2(dpa)Zn(tBu)2]−] (4; 1 equiv) | RT | 42 | 13 | 0 | 3 | 58 | |
| Substoichiometric | ||||||||
| 7 | tBu2Zn (1 equiv) | 75 | 11 | 1 | 0 | 8 | 20 | |
| 8 | tBu2Zn (1 equiv)+[(TMEDA)Na(TMP)] (0.1 equiv) | 75 | 33 | 8 | 0 | 6 | 47 | |
| 9 | tBu2Zn (1 equiv)+[(TMEDA)Na(dpa)]2 (0.1 equiv) | 75 | 52 | 12 | 0 | 7 | 71 | |
| 10 | tBu2Zn (1 equiv)+[[Na(THF)6]+[Zn(tBu)2(dpa)Zn(tBu)2]−] (0.1 equiv) | 75 | 33 | 7 | 0 | 9 | 49 | |
Yields were determined by 1H NMR spectroscopy using hexamethylbenzene (10 mol %) as an internal standard.
