Abstract
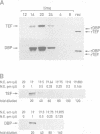
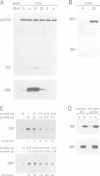
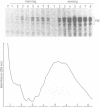
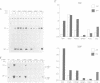
The two highly related PAR basic region leucine zipper proteins TEF and DBP accumulate according to a robust circadian rhythm in liver and kidney. In liver nuclei, the amplitude of daily oscillation has been estimated to be 50-fold and 160-fold for TEF and DBP, respectively. While DBP mRNA expression is the principal determinant of circadian DBP accumulation, the amplitude of TEF mRNA cycling is insufficient to explain circadian TEF fluctuation. Conceivably, daily variations in TEF degradation or nuclear translocation efficiency may explain the discrepancy between mRNA and protein accumulation. In vitro, TEF and DBP bind the same DNA sequences. Yet, in co-transfection experiments, these two proteins exhibit different activation potentials for two reporter genes examined. While TEF stimulates transcription from the albumin promoter more potently than DBP, only DBP is capable of activating transcription efficiently from the cholesterol 7 alpha hydroxylase (C7alphaH) promoter. However, a TEF-DBP fusion protein, carrying N-terminal TEF sequences and the DNA binding/dimerization domain of DBP, enhances expression of the C7alphaH-CAT reporter gene as strongly as wild-type DBP. Our results suggest that the promoter environment, rather than the affinity with which PAR proteins recognize their cognate DNA sequences in vitro, determines the promoter preferences of TEF and DBP.
Full text
PDF


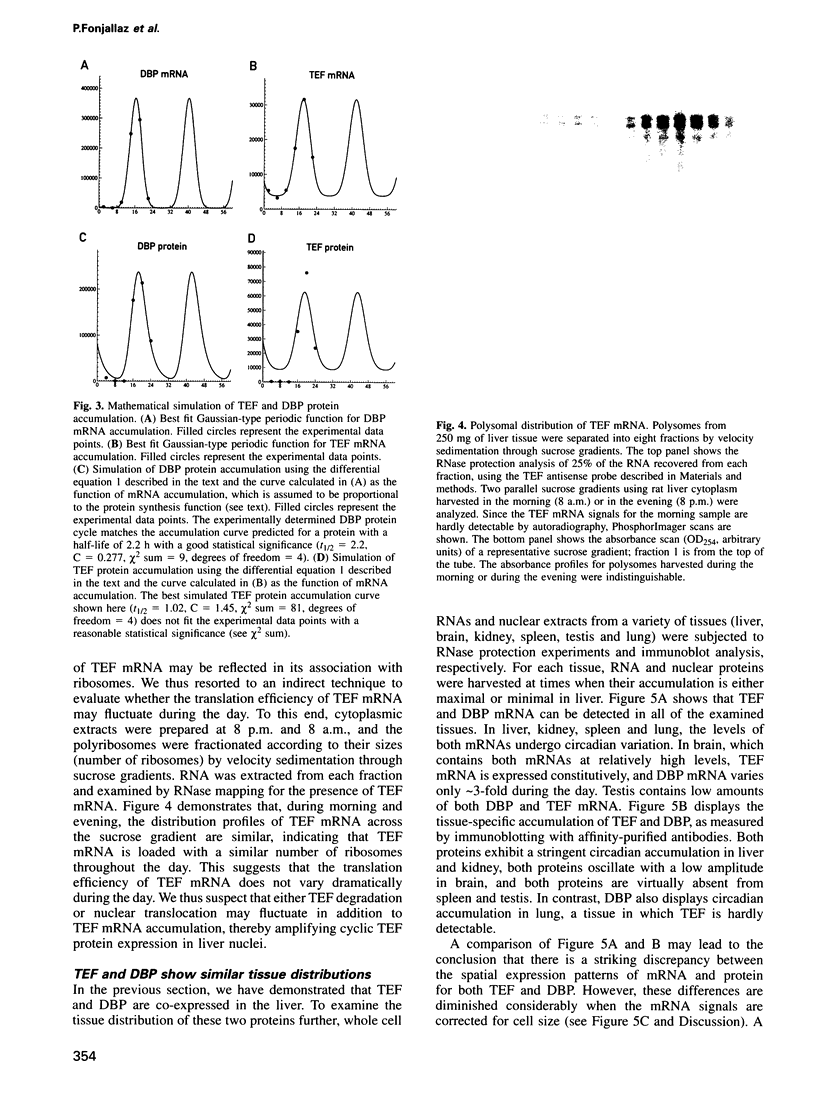








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basler K., Oesch B., Scott M., Westaway D., Wälchli M., Groth D. F., McKinley M. P., Prusiner S. B., Weissmann C. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 1986 Aug 1;46(3):417–428. doi: 10.1016/0092-8674(86)90662-8. [DOI] [PubMed] [Google Scholar]
- Brukner I., Jurukovski V., Savic A. Sequence-dependent structural variations of DNA revealed by DNase I. Nucleic Acids Res. 1990 Feb 25;18(4):891–894. doi: 10.1093/nar/18.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B., Davis D. L. Alternative promoter usage and splicing options result in the differential expression of mRNAs encoding four isoforms of chicken VBP, a member of the PAR subfamily of bZIP transcription factors. Nucleic Acids Res. 1994 Nov 11;22(22):4733–4741. doi: 10.1093/nar/22.22.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Descombes P., Chojkier M., Lichtsteiner S., Falvey E., Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 1990 Sep;4(9):1541–1551. doi: 10.1101/gad.4.9.1541. [DOI] [PubMed] [Google Scholar]
- Descombes P., Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991 Nov 1;67(3):569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Dillon N., Grosveld F. Transcriptional regulation of multigene loci: multilevel control. Trends Genet. 1993 Apr;9(4):134–137. doi: 10.1016/0168-9525(93)90208-y. [DOI] [PubMed] [Google Scholar]
- Drolet D. W., Scully K. M., Simmons D. M., Wegner M., Chu K. T., Swanson L. W., Rosenfeld M. G. TEF, a transcription factor expressed specifically in the anterior pituitary during embryogenesis, defines a new class of leucine zipper proteins. Genes Dev. 1991 Oct;5(10):1739–1753. doi: 10.1101/gad.5.10.1739. [DOI] [PubMed] [Google Scholar]
- Falvey E., Fleury-Olela F., Schibler U. The rat hepatic leukemia factor (HLF) gene encodes two transcriptional activators with distinct circadian rhythms, tissue distributions and target preferences. EMBO J. 1995 Sep 1;14(17):4307–4317. doi: 10.1002/j.1460-2075.1995.tb00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas N. B., Cantwell C. A., Johnson P. F., Burch J. B. DNA-binding specificity of the PAR basic leucine zipper protein VBP partially overlaps those of the C/EBP and CREB/ATF families and is influenced by domains that flank the core basic region. Mol Cell Biol. 1995 Apr;15(4):1923–1932. doi: 10.1128/mcb.15.4.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschlag D., Johnson F. B. Synergism in transcriptional activation: a kinetic view. Genes Dev. 1993 Feb;7(2):173–179. doi: 10.1101/gad.7.2.173. [DOI] [PubMed] [Google Scholar]
- Hunger S. P., Brown R., Cleary M. L. DNA-binding and transcriptional regulatory properties of hepatic leukemia factor (HLF) and the t(17;19) acute lymphoblastic leukemia chimera E2A-HLF. Mol Cell Biol. 1994 Sep;14(9):5986–5996. doi: 10.1128/mcb.14.9.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunger S. P., Ohyashiki K., Toyama K., Cleary M. L. Hlf, a novel hepatic bZIP protein, shows altered DNA-binding properties following fusion to E2A in t(17;19) acute lymphoblastic leukemia. Genes Dev. 1992 Sep;6(9):1608–1620. doi: 10.1101/gad.6.9.1608. [DOI] [PubMed] [Google Scholar]
- Iyer S. V., Davis D. L., Seal S. N., Burch J. B. Chicken vitellogenin gene-binding protein, a leucine zipper transcription factor that binds to an important control element in the chicken vitellogenin II promoter, is related to rat DBP. Mol Cell Biol. 1991 Oct;11(10):4863–4875. doi: 10.1128/mcb.11.10.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatib Z. A., Inaba T., Valentine M., Look A. T. Chromosomal localization and cDNA cloning of the human DBP and TEF genes. Genomics. 1994 Sep 15;23(2):344–351. doi: 10.1006/geno.1994.1510. [DOI] [PubMed] [Google Scholar]
- Krylov D., Mikhailenko I., Vinson C. A thermodynamic scale for leucine zipper stability and dimerization specificity: e and g interhelical interactions. EMBO J. 1994 Jun 15;13(12):2849–2861. doi: 10.1002/j.1460-2075.1994.tb06579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery D. J., Schibler U. Circadian transcription of the cholesterol 7 alpha hydroxylase gene may involve the liver-enriched bZIP protein DBP. Genes Dev. 1993 Oct;7(10):1871–1884. doi: 10.1101/gad.7.10.1871. [DOI] [PubMed] [Google Scholar]
- Lichtsteiner S., Wuarin J., Schibler U. The interplay of DNA-binding proteins on the promoter of the mouse albumin gene. Cell. 1987 Dec 24;51(6):963–973. doi: 10.1016/0092-8674(87)90583-6. [DOI] [PubMed] [Google Scholar]
- Mueller C. R., Maire P., Schibler U. DBP, a liver-enriched transcriptional activator, is expressed late in ontogeny and its tissue specificity is determined posttranscriptionally. Cell. 1990 Apr 20;61(2):279–291. doi: 10.1016/0092-8674(90)90808-r. [DOI] [PubMed] [Google Scholar]
- Rusconi S., Severne Y., Georgiev O., Galli I., Wieland S. A novel expression assay to study transcriptional activators. Gene. 1990 May 14;89(2):211–221. doi: 10.1016/0378-1119(90)90008-f. [DOI] [PubMed] [Google Scholar]
- Schmidt E. E., Schibler U. Cell size regulation, a mechanism that controls cellular RNA accumulation: consequences on regulation of the ubiquitous transcription factors Oct1 and NF-Y and the liver-enriched transcription factor DBP. J Cell Biol. 1995 Feb;128(4):467–483. doi: 10.1083/jcb.128.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. E., Schibler U. High accumulation of components of the RNA polymerase II transcription machinery in rodent spermatids. Development. 1995 Aug;121(8):2373–2383. doi: 10.1242/dev.121.8.2373. [DOI] [PubMed] [Google Scholar]
- Tian J. M., Schibler U. Tissue-specific expression of the gene encoding hepatocyte nuclear factor 1 may involve hepatocyte nuclear factor 4. Genes Dev. 1991 Dec;5(12A):2225–2234. doi: 10.1101/gad.5.12a.2225. [DOI] [PubMed] [Google Scholar]
- Wuarin J., Falvey E., Lavery D., Talbot D., Schmidt E., Ossipow V., Fonjallaz P., Schibler U. The role of the transcriptional activator protein DBP in circadian liver gene expression. J Cell Sci Suppl. 1992;16:123–127. doi: 10.1242/jcs.1992.supplement_16.15. [DOI] [PubMed] [Google Scholar]
- Wuarin J., Schibler U. Expression of the liver-enriched transcriptional activator protein DBP follows a stringent circadian rhythm. Cell. 1990 Dec 21;63(6):1257–1266. doi: 10.1016/0092-8674(90)90421-a. [DOI] [PubMed] [Google Scholar]
- Wuarin J., Schibler U. Physical isolation of nascent RNA chains transcribed by RNA polymerase II: evidence for cotranscriptional splicing. Mol Cell Biol. 1994 Nov;14(11):7219–7225. doi: 10.1128/mcb.14.11.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]