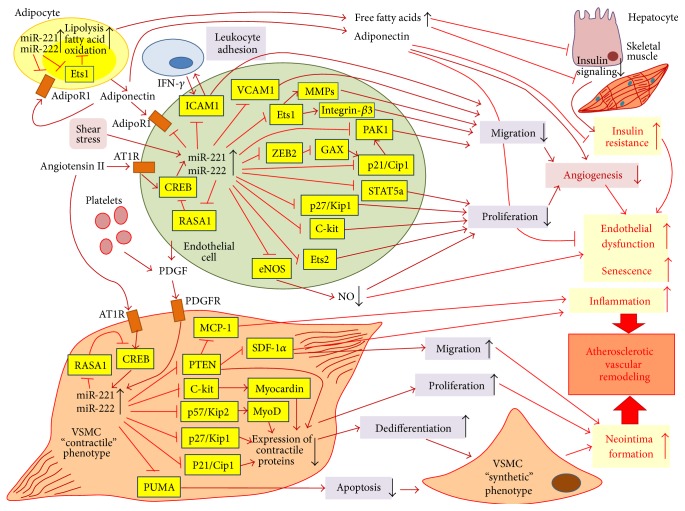
Figure 2.
Effects of miR-221/222 on vascular endothelium and vascular smooth muscle cells (VSMCs) in atherosclerotic vascular remodeling. In arterial endothelial cells (ECs), expression of miR-221/222 could be upregulated by angiotensin II and shear stress. miR-221/222 are able to positively regulate expression through suppression of RAS p21 protein activator 1 (RASA1), an inhibitor of CREB (cAMP response element-binding protein) that drives angiotensin II induced expression of both miRNAs. Increased levels of miR-221/222 suppress angiogenic activation of quiescent terminally differentiated ECs through inhibiting endothelial proliferation and migration. Proliferation is suppressed via negative regulatory effects of the miR-221/222 cluster on several key genes such as cyclin-dependent kinase cell cycle regulators p21Cip1 and p27Kip1, transcription factors Ets1 and Ets2, signal transducer and activator STAT5a, and receptor for mast/stem cell growth factor c-kit. Notably, miR-221/222 downregulate expression of endothelial NO-synthase (eNOS) that lead to lowered production of nitric oxide (NO), an important modulator of function and proliferation of vascular ECs. Decreased NO production contributes to endothelial dysfunction and promotes EC senescence. miR-221/222 could downregulate p21Cip1 either directly or through blocking of ZEB2 (zinc finger E-box binding homeobox 2), which represses translation of mesenchyme homeobox 2 (MEOX2 or GAX), a transcriptional activator of p21Cip1. The miR-221/222 cluster attenuates EC migration by suppressing endothelial production of matrix metalloproteinases (MMPs) and several key adhesion modulators such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), integrin-β3, and serine/threonine-protein kinase PAK-1. The miR-221/222 cluster could probably diminish endothelial expression of adiponectin receptor AdipoR1. Adiponectin is produced by adipocytes and plays protective role for ECs by preventing endothelial dysfunction. In obesity, miR-221/222 are upregulated in the adipose tissue causing activation of lipid catabolism (lipolysis and fatty cell oxidation) through inhibition of Ets1, a transcription factor that controls expression of fatty acid synthase and other lipid-synthesizing enzymes. As a result, adipocytes release increased amounts of free fatty acids to blood that inhibit insulin signaling in liver and skeletal muscle inducing peripheral insulin resistance. Insulin resistance contributes to endothelial dysfunction. In VSMCs, expression of miR-221/222 is stimulated by angiotensin II and platelet-derived growth factor (PDGF) that is secreted by activated platelets and ECs in response to vascular injury. Upregulation of these miRNAs supports proliferation and increase mobility of VSMCs. In VSMCs, miR-221/222 inhibit several regulatory factors such as those of p21Cip1, p27Kip1, p57Kip2, c-kit, and phosphatase and tensin homolog (PTEN) that are crucial for differentiation and establishment of the contractile phenotype of VSMCs. p57Kip2 and c-kit activate MyoD and myocardin, two key transcription factors involved in myogenesis. Indeed, miR-221/222-dependent downregulation of expression of SMC-specific contractile proteins causes VSMC dedifferentiation and switch from the “contractile” to “synthetic” phenotype. By suppressing PTEN, miR-221/222 induce expression of several proinflammatory chemokines such as monocyte chemotactic protein-1 (MCP-1) and stromal cell-derived factor 1α (SDF-1α) that attract proinflammatory lymphocytes, dendritic cells, and macrophages to the inflamed site, for example, to the atherosclerotic plaque. In addition, miR-221/222 downregulate PUMA (p53 upregulated modulator of apoptosis), a critical apoptotic inducer thereby preventing apoptosis of VSMCs. Finally, dedifferentiated VSMCs become involved in neointima formation, an essential stage in atherosclerosis-associated remodeling of the arterial wall.