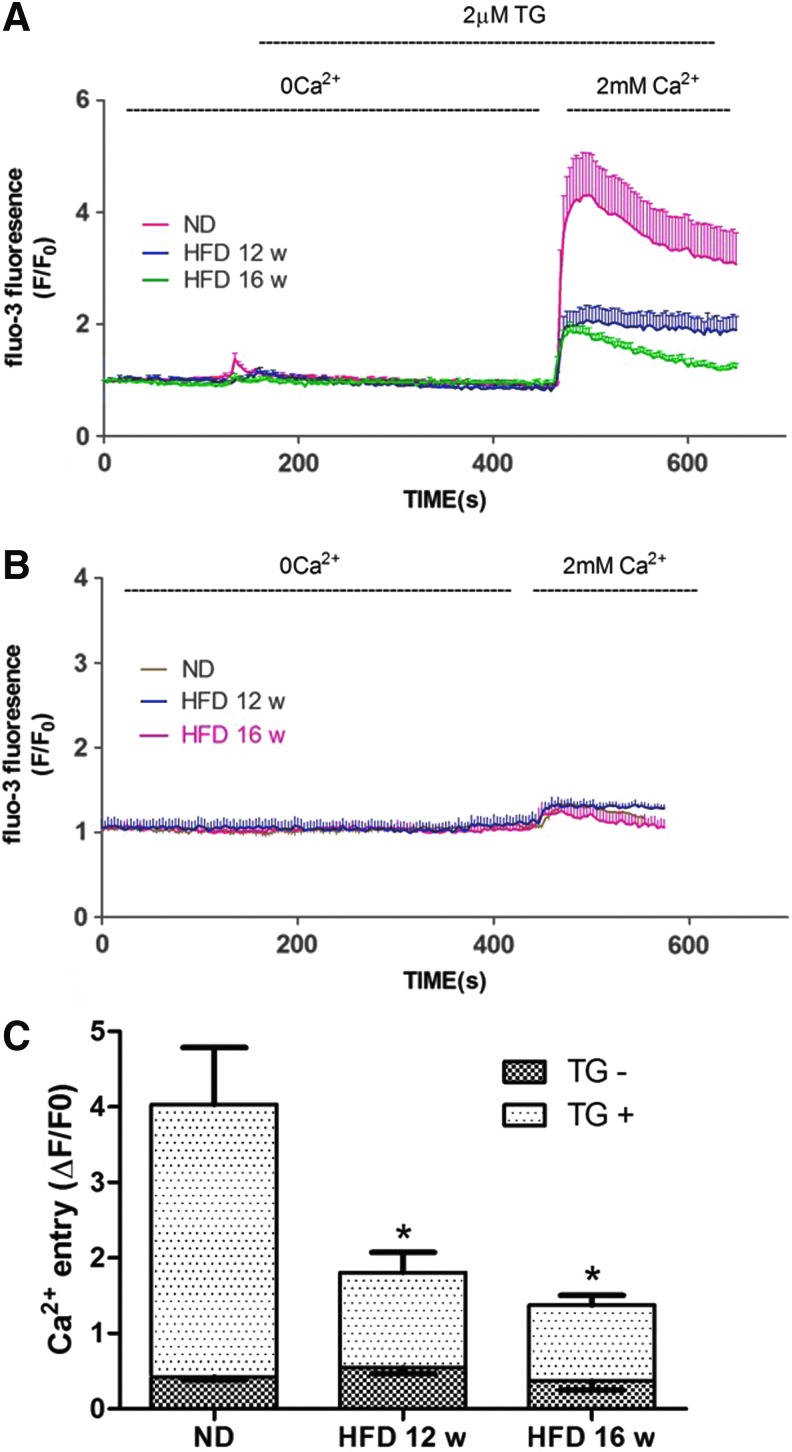
FIG. 2.
Decreased store-operated calcium entry (SOCE) amplitude in HFD-EPCs. (A) Ca2+ was measured through confocal microscopy of cells stained with the fluorescent dye, Fluo3. During exposure to PSS without Ca2+, Thapsigargin (TG) (2 μM) was used to deplete intracellular Ca2+ stores, after which Ca2+ (2 mM) was added back to the bathing medium, thereby eliciting a rise in [Ca2+]i due to SOCE. The red (13 cells), blue (12 cells), and green (15 cells) traces depict representative time courses of [Ca2+]i changes in EPCs isolated from ND, 12-, and 16-week HFD mice, respectively. (B) In the absence of TG stimulation, Ca2+ readdition resulted in only miniscule Ca2+ entry in EPCs. The brown (15 cells), blue (10 cells), and red (14 cells) traces depict representative time courses of [Ca2+]i changes in EPCs isolated from ND, 12-, and 16-week HFD mice, respectively. (C) Quantification of Ca2+ entry amplitude. Cells in each group were harvested from three different mice. The data are presented as the mean±SE. ND=41, 12-week HFD=35, 16-week HFD=38, ND without TG=38, 12-week HFD without TG=30, 16-week HFD without TG=35; *P<0.05 versus ND-EPCs.