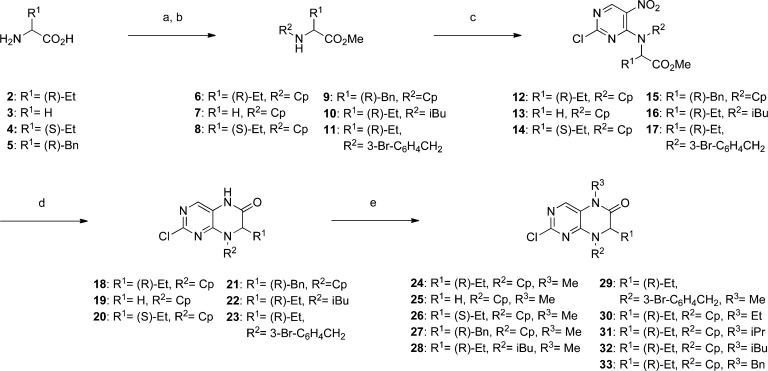
Scheme 1.
Reagents and conditions: (a) SOCl2, MeOH, 0 °C to reflux, 16 h; (b) cyclopentanone, isobutyraldehyde or 3-bromobenzaldehyde, NaBH(OAc)3, NaOAc, CH2ClCH2Cl, RT, 16 h; (c) 2,4-dichloro-5-nitropyrimidine, K2CO3, acetone, RT, 16 h; (d) Fe, AcOH, 70 °C, 1 h, 100 °C, 4–5 h; (e) alkyl iodide or benzyl bromide, NaH, DMF, 0 °C to RT, 3−16 h. Cp = cyclopentyl.