Table 1. Structure–Activity Relationships of the R1, R2, R3, R4, and X Groups; Cp = Cyclopentyl.
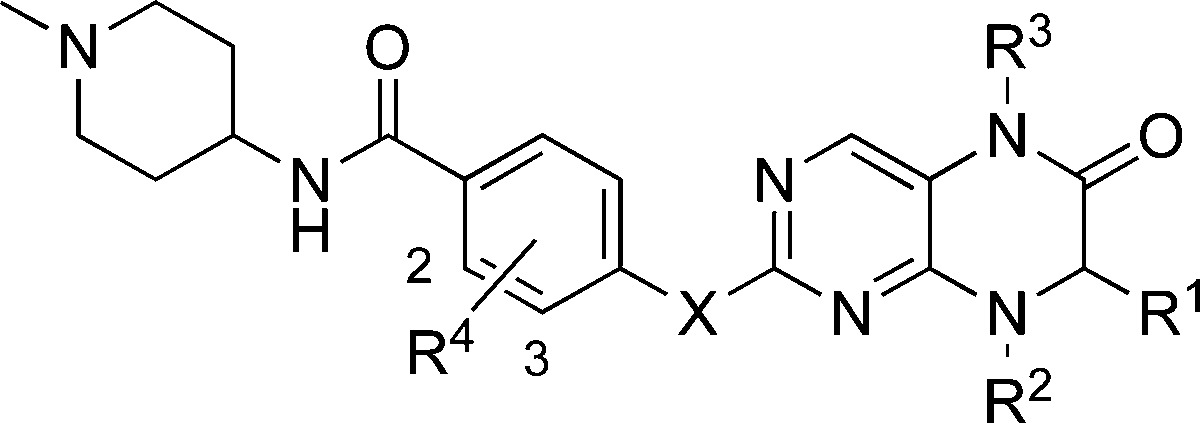
| compd | R1 | R2 | R3 | R4 | X | BRD4 Ki (nM)a | PLK1 Ki (nM)a |
|---|---|---|---|---|---|---|---|
| BI-2536 | (R)-Et | Cp | Me | 3-OMe | NH | 56 ± 9 | 0.22 ± 0.01 |
| 39a | (R)-Et | Cp | H | 3-OMe | NH | 7950 ± 1550 | ND |
| 39b | (R)-Et | Cp | Et | 3-OMe | NH | 470 ± 10 | 0.91 ± 0.13 |
| 39c | (R)-Et | Cp | iPr | 3-OMe | NH | 4500 ± 300 | ND |
| 39d | (R)-Et | Cp | iBu | 3-OMe | NH | >10000 | ND |
| 39e | (R)-Et | Cp | Bn | 3-OMe | NH | >10000 | ND |
| 39f | H | Cp | Me | 3-OMe | NH | 1400 ± 400 | ND |
| 39g | (S)-Et | Cp | Me | 3-OMe | NH | 54 ± 4 | 0.42 ± 0.04 |
| 39h | (R)-Bn | Cp | Me | 3-OMe | NH | >10000 | 4.20 ± 0.99 |
| 39i | (R)-Et | iBu | Me | 3-OMe | NH | 970 ± 30 | 6.95 ± 1.63 |
| 39j | (R)-Et | 3-Br-C6H4CH2 | Me | 3-OMe | NH | 8.7 ± 1.3 | 5.80 ± 0.99 |
| 39k | (R)-Et | Cp | Me | H | NH | 60 ± 7 | ND |
| 39l | (R)-Et | Cp | Me | 2-OMe | NH | 37 ± 5 | 0.52 ± 0.01 |
| 39m | (R)-Et | Cp | Me | 3-F | NH | 46 ± 2 | ND |
| 39n | (R)-Et | Cp | Me | 3-OiBu | NH | 29 ± 9 | ND |
| 39o | (R)-Et | Cp | Me | 3-OBn | NH | 100 ± 0 | ND |
| 39p | (R)-Et | Cp | Me | 3-OCp | NH | 27 ± 8 | 0.84 ± 0.01 |
| 39q | (R)-Et | Cp | Me | 3-OMe | O | 99 ± 11 | 240 ± 14 |
Data represent the mean and standard deviation of two independent experiments. ND = not determined.
