Fig. S2.
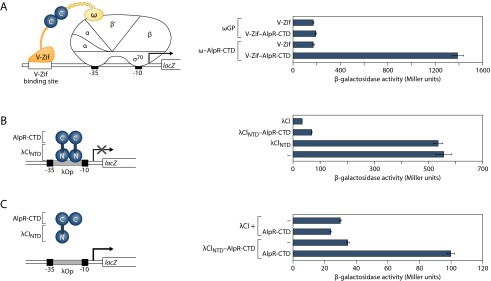
The predicted CTD of AlpR contains a dimerization determinant. (A) Bacterial two-hybrid analysis demonstrates interaction between AlpR-CTDs. Contact between AlpR-CTDs (denoted as “C”) fused to both the ω subunit of E. coli RNAP and to V-Zif activates transcription from the test promoter driving expression of lacZ. The diagram depicts test promoter placZif1, which bears a V-Zif-binding site positioned upstream of the lac core promoter. Effect of the ω–Gal11P (indicated ωGP), or ω–AlpR-CTD fusion protein on expression of the lacZ reporter in the presence of either the unfused V-Zif or the V-Zif–AlpR-CTD fusion protein. KDZif1ΔZ cells harboring compatible plasmids directing the IPTG-inducible synthesis of the indicated proteins were grown in the presence of 50 µM IPTG and assayed for β-galactosidase activity. This finding indicated that only the AlpR-CTD fusion proteins are capable of interacting. (B) The AlpR-CTD can functionally substitute for the dimerization domain of the CI protein from bacteriophage λ (λCI). FW123 cells containing a plasmid directing the IPTG-dependent synthesis of either the N-terminal domain and linker of λCI (λCINTD), λCI, or the λCINTD–AlpR-CTD fusion protein were grown in the presence of 5 µM IPTG and assayed for β-galactosidase activity. In this system the AlpR-CTD functionally replaces the dimerization domain of λCI. Occupancy of the λ operator (located between the promoter −35 and −10 elements) by λCI prevents RNAP from binding the promoter and represses transcription. (C) Ectopic synthesis of the AlpR-CTD specifically interferes with the ability of the λCINTD–AlpR-CTD fusion protein to bind the λ operator in E. coli, resulting in de-repression of the test promoter.
