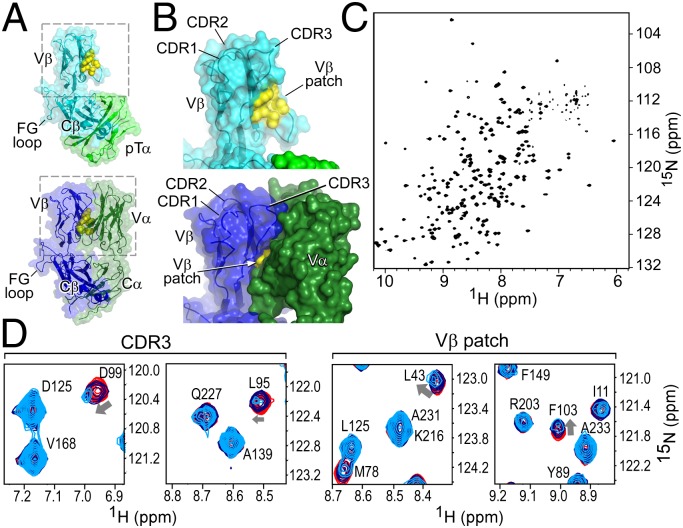
Fig. 1.
The structure of the β-subunit when incorporated into pTαβ or TCRαβ heterodimers suggests that the preTCR has ligand binding properties. (A and B) Structures of the (Upper) pTα/LC13β preTCR [Protein Data Bank (PDB) ID code 3OF6] and (Lower) N15αβTCR (PDB ID code 1NFD) heterodimers. (A) The overall fold of β remains consistent within pTαβ compared with within TCRαβ. (B) Highlight of Vβ domain and CDR loops within pTαβ and αβTCR. The hydrophobic Vβ patch, which is exposed in pTαβ but not in TCRαβ, is shown in yellow. (C) TROSY-HSQC spectrum of N15β (with backbone residue assignments overlaid in Fig. S1B) showing spectral dispersion consistent with the β sheet-rich fold of the β-subunit. (D) Select regions of overlaid TROSY-HSQC spectra of 200 μM 1H-15N N15β alone (red) and with the addition of 200 (blue) or 500 μM (cyan) unlabeled VSV8/Kb. Chemical shift changes are highlighted for residues D99 and L95 of CDR3 and L43 and F103 of the Vβ patch. Note the lack of changes in C-domain residues, for example, D125, A139, V168 or A233.