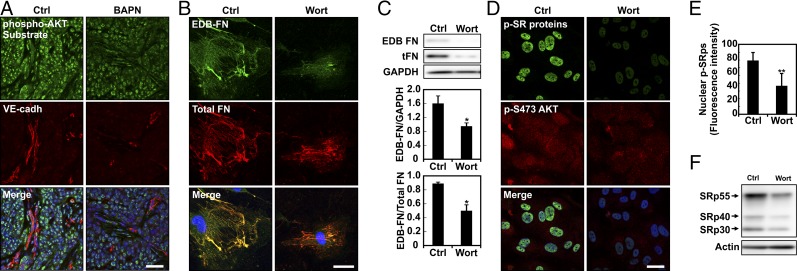
Fig. 6.
PI3K-AKT signaling is required for splicing of EDB-FN. (A) Representative image of frozen tissue sections stained for phosphorylated AKT substrates and VE-cadherin showing that the BAPN treatment influences PI3K/AKT signaling pathway activation. (Scale bar, 50 μm.) (B) Confocal images of EDB-FN and total FN on EC cells plated on 10-kPa substrates and kept in culture for 24 h with or without (Ctrl) wortmannin (Wort). (C) Western blot of whole protein extract from EC cells seeded on 10-kPa substrates showing that wortmannin (Wort)-mediated inhibition of PI3K decreases EDB-FN compared with control. Corresponding densitometric quantification showing both EDB-FN normalized against GAPDH content and the ratio of EDB-FN to total FN. (D) Representative image of ECs stained for phosphorylated SR proteins and AKT phosphorylation on Ser-473 showing that 1-h treatment with wortmannin prevents both SR protein and AKT phosphorylation. (Scale bar, 30 μm.) (E) Corresponding quantification of SR protein phosphorylation computed from at least 18 different fields of view from three independent experiments. (F) Western blot of whole protein extract from EC cells plated on stiff (E = 10 kPa) substrates showing that wortmannin (Wort)-mediated inhibition of PI3K lowered SR protein phosphorylation compared to control (Ctrl). Actin was used as a loading control. Plots are mean ± SE, Student t test: *P < 0.05, **P < 0.01. Images were acquired with identical exposure settings.