Figure 1.
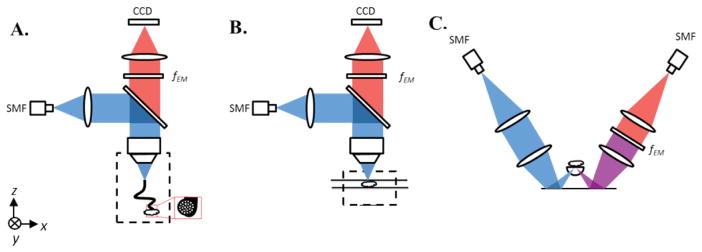
(A) HRFM setup. Excitation light from a 405-nm fiber-coupled diode laser is deflected by a dichroic beam splitter and focused into a coherent imaging fiber bundle. The fiber bundle transmits the light to the sample that is placed in contact with the distal end of the bundle. Emission light travels back from the sample through the bundle, is transmitted through the dichroic beam splitter and a long-pass emission filter (fEM), and is then imaged onto a CCD. (B) WFM setup. Excitation light travels the same path as in the HRFM setup, but is focused directly at the sample surface, instead of through a fiber bundle. Fluorescence emission is collected by a CCD detector array, as in the HRFM. (C) Dual-axis confocal (DAC) microscope setup. Excitation light from a 405-nm fiber-coupled diode laser is collimated and focused into tissue through a hemispherical fused-silica solid-immersion lens (SIL). Fluorescence emission is collected along an off-axis but symmetric optical path that includes a long-pass emission filter (fEM) and a single-mode collection fiber (SMF) that acts as a detection pinhole, followed by a PMT detector.
