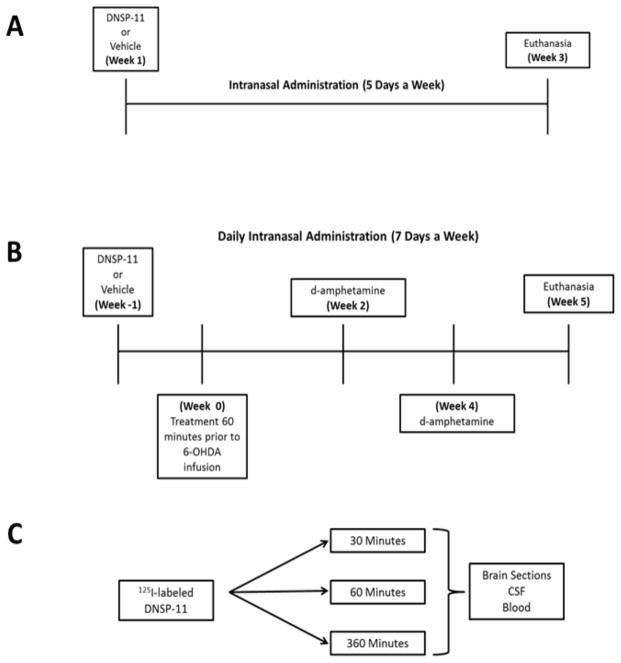
Figure 2. Experimental timeline for all intranasal studies.
Three major studies designs were used to determine the efficacy of repeated intranasally administered DNSP-11’s effects on the nigrostriatal system in both normal and unilateral 6-OHDA- lesioned rats using isoflurane anesthesia. (A) To determine the efficacy of our intranasal delivery method and adequate dosing, normal rats were subjected to repeated intranasal administration of various doses of DNSP-11, 5 days a week for 3 weeks (21 days). (B) Utilizing the determined dosage from experiment A, the efficacy of intranasally administered DNSP-11’s effects on the nigrostriatal system were examined in a unilateral 6-OHDA (right hemisphere) lesion model of PD where rats were treated with either vehicle or DNSP-11 daily, 1 week (7 days) prior to unilateral 6-OHDA infusion and 5 weeks (35 ± 3 days) post-surgery. Prior to the start of the study baseline d-amphetamine rotation data were determined and at both 2 and 4 weeks post unilateral 6-OHDA infusion to assess lesion severity. (A–B) For experiments A and B, researchers were blinded to the treatments throughout the entirety of the studies. (C) Tracer studies examined 125I-labeled DNSP-11’s distribution at 30, 60, and 360 minutes after the start of the first intranasal dose in blood, CSF and the CNS.