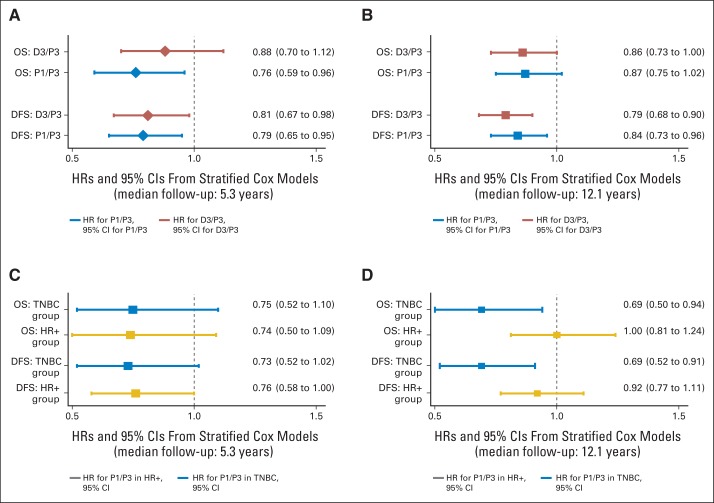
Fig 3.
Disease-free survival (DFS) and overall survival (OS) hazard ratios (HRs) and 95% CIs for weekly paclitaxel (P1) and docetaxel every 3 weeks (D3) compared with paclitaxel every 3 weeks (P3) in overall population (A, B) and in patients with triple-negative breast cancer (TNBC) and hormone receptor–positive/human epidermal growth factor receptor 2–nonoverexpressing disease (HR+; C, D), including the prior report and current report.