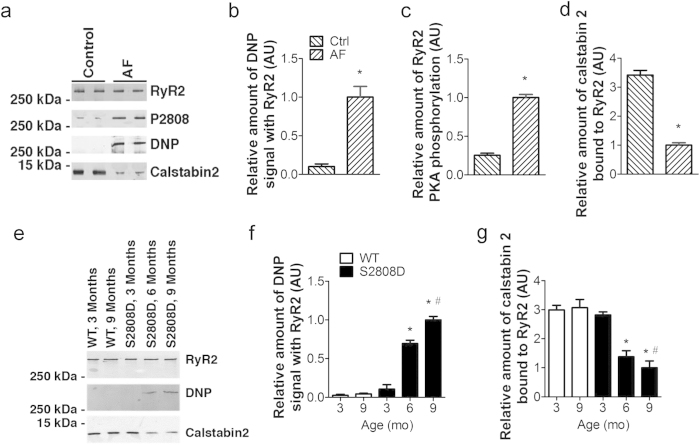
Figure 1. Increased oxidation of the atrial RyR2 complex in patients with AF and in RyR2-S2808D+/+ mice.
(a) post-translational modifications of the RyR2 complex in right atrial (RA) tissue of patients with AF and normal ventricular function or controls. RA appendage tissue was obtained at the time of cardiac surgery from patients with chronic AF (>6 months; n = 10), and patients in sinus rhythm (n = 10). To determine RyR2 channel oxidation, the carbonyl groups in the protein side chains of immunoprecipitated RyR2 were derivatized to (DNP) by reaction with 2,4-dinitrophenylhydrazine. The DNP (2,4-dinitrophenylhydrazone) signal associated with RyR2 was determined by anti-DNP antibody. (b–d), Quantification of DNP signal (b), PKA hyperphosphorylation (c), and calstabin 2 bound to RyR2 (d) in human atrial samples. *p < 0.01 vs control. Error bars represent s.e.m. (e) Post-translational modifications of the RyR2 complex in atrial samples from WT and RyR2-S2808D+/+ mice. (f) and (g), Quantification of DNP signal (f) and calstabin 2 bound to RyR2 (g); atrial samples were obtained from at least 5 mice in each group. AU: arbitrary units. All data are shown as mean ± s.e.m. * and **: p < 0.05 and 0.01 vs 3-month-old group; #: p < 0.05 vs WT.