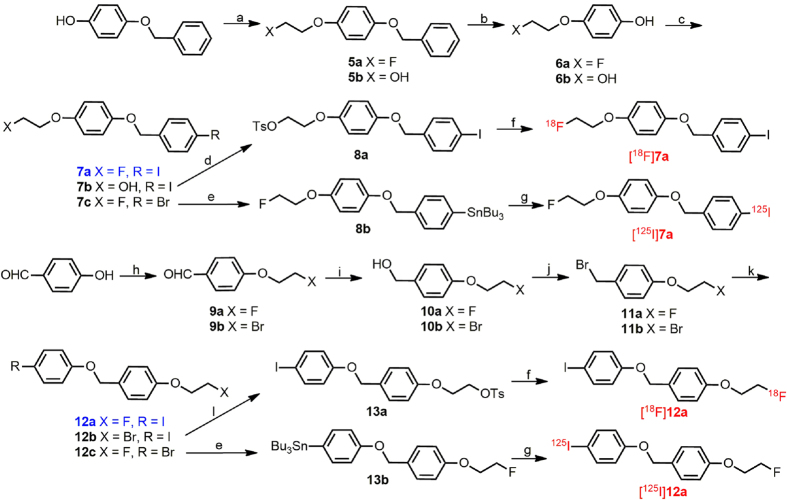
Figure 4. Chemical synthesis routes.
Reagents and conditions: (a) 1-bromo-2-fluoroethane or 2-chloroethanol, KOH, EtOH, reflux; (b) 10% Pd/C, 1 atm H2, 50 °C; (c) 1-(bromomethyl)-4-iodobenzene or 1-bromo-4-(bromomethyl)benzene, K2CO3, DMF, 90 °C; (d) TsCl, CH2Cl2, Et3N, r.t.; (e) (Bu3Sn)2, (PPh3)4Pd, toluene, Et3N, reflux; (f)18F–, K2CO3, Kryptofix-2.2.2, acetonitrile, 100 °C; (g) [125I]NaI, HCl (1 M), H2O2 (3%); (h) 1-bromo-2-fluoroethane or 1,2-dibromoethane, K2CO3, DMF, 90 oC; (i) NaBH4, MeOH, 0 °C; (j) PBr3, CH2Cl2, r.t.; (k) 4-iodophenol or 4-bromophenol, K2CO3, DMF, 90 °C; (l) AgOTs, MeCN, reflux.