Scheme 1.
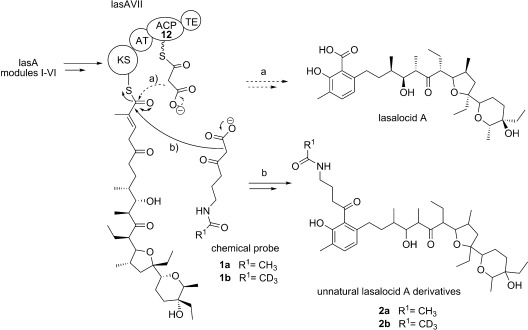
a) Late stages in the biosynthesis of lasalocid A by the lasA PKS: malonyl-ACP decarboxylative condensation with the last KS-bound intermediate, followed by aromatization and thioester hydrolysis, leads to natural product formation. b) The competitive decarboxylative condensation of the carba(dethia) N-acetyl cysteamine probes 1 a,b11b with the same advanced intermediate leads to the formation of the lasalocid A unnatural derivatives 2 a,b. ACP=acyl carrier protein, AT=acyl transferase, KS=ketosynthase, TE=thioesterase.
