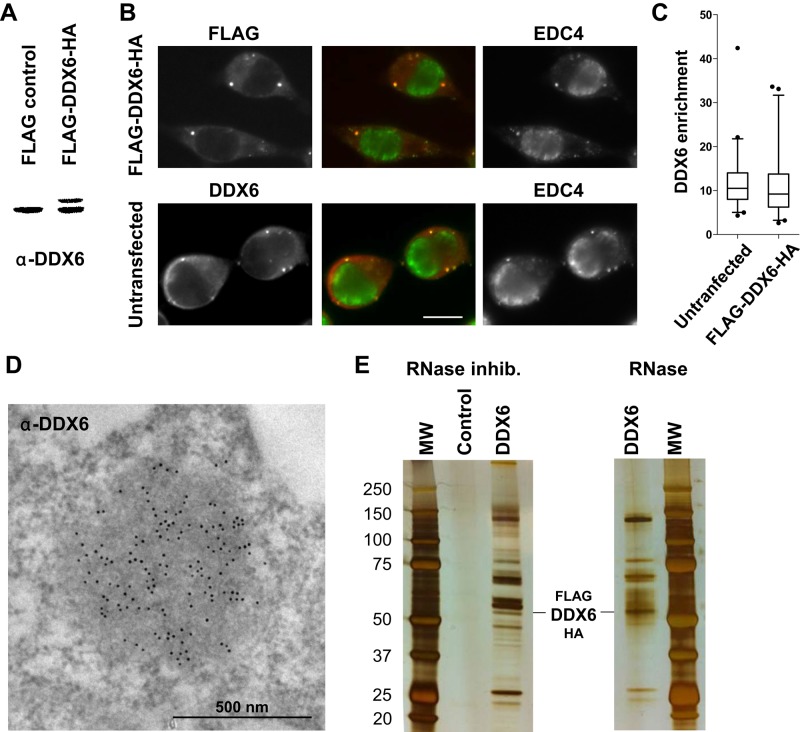
FIGURE 1:
Purification of DDX6 complexes by TAP-tag. (A) HEK293 cells were transfected with a FLAG-DDX6-HA or an empty plasmid as a control. After 48 h, proteins were analyzed by Western blotting with anti-DDX6 antibodies. (B) In parallel, transfected cells were stained with anti-FLAG (red) and anti-EDC4 (green) antibodies to detect the exogenous DDX6 protein and P-bodies, respectively. For comparison, untransfected cells were stained with anti-DDX6 and anti-EDC4 antibodies. Owing to the round shape of the cells, the maximal projection of a z-series of images is shown to better visualize P-bodies. Scale bar: 10 μm. (C) DDX6 enrichment in P-bodies with respect to the surrounding cytoplasm (n = 94 and 90 for transfected and untransfected samples, respectively) was quantified from single-plane images. (D) Endogenous DDX6 was detected in untransfected HEK293 cells by immunoelectron microscopy, as previously described (Souquere et al., 2009). One P-body highly enriched in DDX6 is shown. (E) Cells transfected with a FLAG-DDX6-HA or an empty plasmid were lysed in the presence of RNase inhibitor or RNase, and DDX6 complexes were purified by TAP-tag. Proteins were migrated on a denaturing gel along with a molecular weight marker (MW) and silver stained.