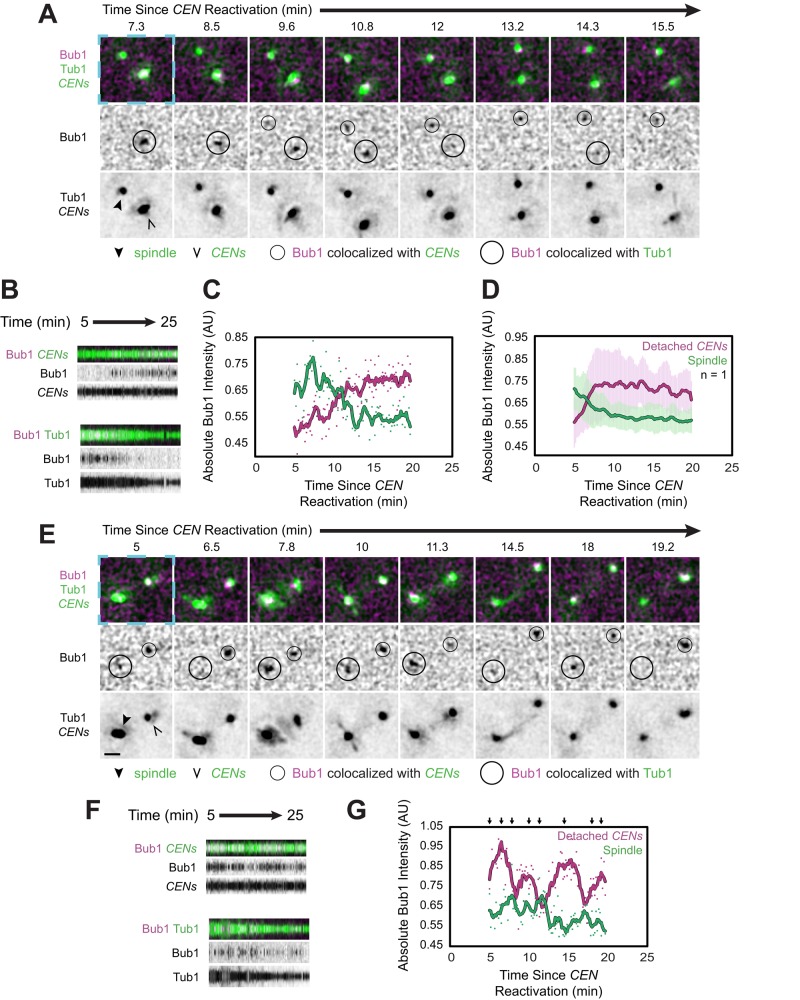
FIGURE 7:
Bub1 localization to spindles and de novo assembled kinetochores. Cells expressing Bub1-3×mCherry, GFP-Tub1, and TetR-GFP and bearing MET3pr-CDC20 and GAL1pr-CEN3-TetOs were grown for 3 h at 25°C in YP medium plus 2 mM methionine, 2% raffinose, and 2% galactose to synchronize cells in metaphase and inactivate CEN3. The cells were then released into synthetic medium plus 2 mM methionine containing 2% glucose at 25°C to reactivate CEN3 and then analyzed by epifluorescence microscopy beginning 5 min later, taking z-stacks every 10 s. Representative deconvolved, Gaussian-filtered MIPs are shown in A and E. Scale bars, 1 μm. The blue dashed boxes surrounding the first frames of A and E highlight the corresponding regions of the expanded views shown in Supplemental Figure S7, B and C, and Supplemental Videos S8 and S9. (A) Time series showing the accumulation of Bub1-3×mCherry at the reactivated centromeres and its disappearance from the spindle, marked by GFP-Tub1 (Supplemental Video S8). (B) Kymographs from the cell in A, made from 15 × 15 boxes approximately centered on the centromeres (top) and 19 × 19 boxes approximately centered on the spindle (bottom). (C) Absolute (i.e., not normalized) intensity of Bub1 at the centromeres and spindle shown in A and B. (D) Absolute intensity of Bub1 at the centromeres and spindle, averaged across the cells in which Bub1 recruitment to the centromeres was accompanied by its disappearance from the spindle. Error bars represent SD. (E) Time series of a cell in which Bub1 alternately accumulated at the centromeres or the spindle and its accumulation at one site was accompanied by its disappearance from the other site (Supplemental Video S9). (F) Kymographs from the cell in E, made as in B. (G) Absolute intensity of Bub1 (not normalized) at the centromeres and spindle shown in D and E. Arrows above the graph refer mark the frames depicted in E. Color-coded sliding window averages of five frames are also shown.