Abstract
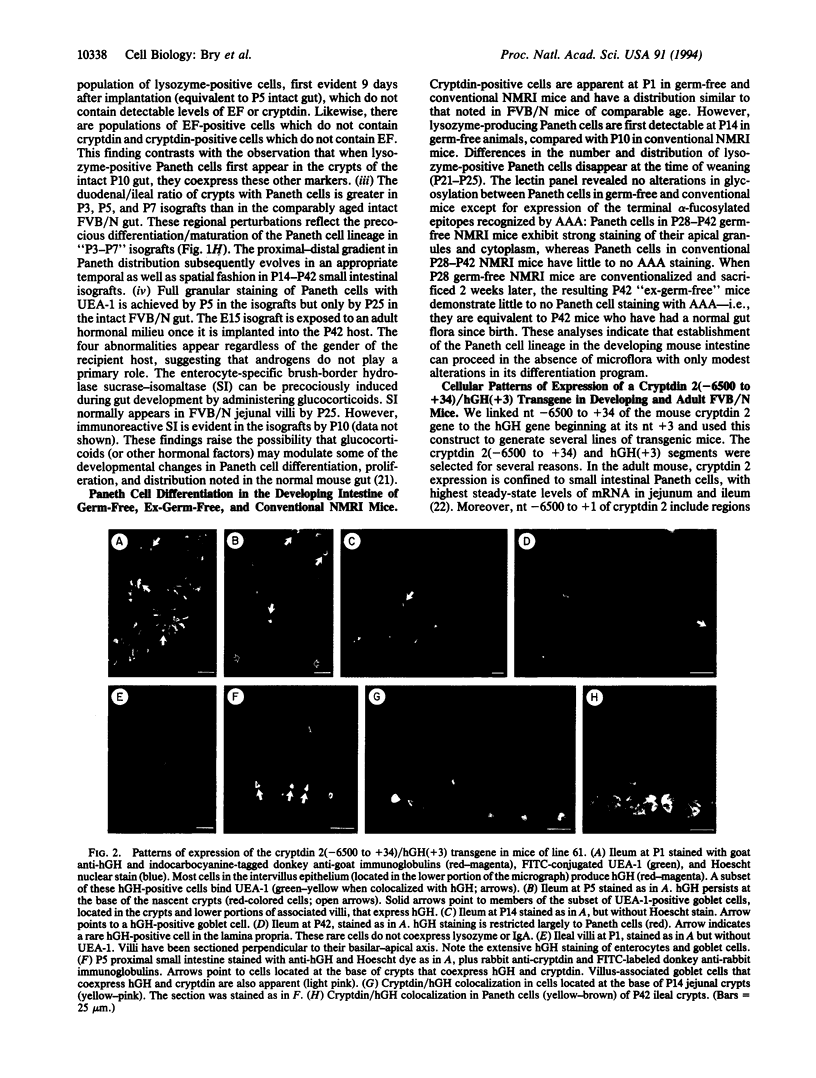
Paneth cells represent one of the four major epithelial lineages in the mouse small intestine. It is the only lineage that migrates downward from the stem-cell zone located in the lower portion of the crypt of Lieberkühn to the crypt base. Mature Paneth cells release growth factors, digestive enzymes, and antimicrobial peptides from their apical secretory granules. Some of these factors may affect the crypt stem cell, its transit-cell descendants, differentiating villus-associated epithelial lineages, and/or the gut microflora. We used single and multilabel immunocytochemical methods to study Paneth cell differentiation during and after completion of gut morphogenesis in normal, gnotobiotic, and transgenic mice as well as in intestinal isografts. This lineage emerges coincident with cytodifferentiation of the fetal small intestinal endoderm, formation of crypts from an intervillus epithelium, and establishment of a stem-cell hierarchy. The initial differentiation program involves sequential expression of cryptdins, a phospholipase A2 (enhancing factor), and lysozyme. A dramatic increase in Paneth cell number per crypt occurs during postnatal days 14-28, when crypts proliferate by fission. Accumulation of fucosylated and sialylated glycoconjugates during this period represents the final evolution of the lineage's differentiation program. Establishment of this lineage is not dependent upon instructive interactions from the microflora. Transgenic mice containing nucleotides -6500 to +34 of the Paneth cell-specific mouse cryptdin 2 gene linked to the human growth hormone gene beginning at its nucleotide +3 inappropriately express human growth hormone in a large population of proliferating and nonproliferating cells in the intervillus epithelium up to postnatal day 5. Transgene expression subsequently becomes restricted to the Paneth cell lineage in the developing crypt. Cryptdin 2 nucleotides -6500 to +34 should be a useful marker of crypt morphogenesis and a valuable tool for conducting gain-of-function or loss-of-function experiments in Paneth cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Nafussi A. I., Wright N. A. Cell kinetics in the mouse small intestine during immediate postnatal life. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;40(1):51–62. doi: 10.1007/BF02932850. [DOI] [PubMed] [Google Scholar]
- Breitman M. L., Rombola H., Maxwell I. H., Klintworth G. K., Bernstein A. Genetic ablation in transgenic mice with an attenuated diphtheria toxin A gene. Mol Cell Biol. 1990 Feb;10(2):474–479. doi: 10.1128/mcb.10.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert R., Pothier P. Migration of fetal intestinal intervillous cells in neonatal mice. Anat Rec. 1990 Jun;227(2):199–206. doi: 10.1002/ar.1092270208. [DOI] [PubMed] [Google Scholar]
- Cheng H., Bjerknes M. Whole population cell kinetics and postnatal development of the mouse intestinal epithelium. Anat Rec. 1985 Apr;211(4):420–426. doi: 10.1002/ar.1092110408. [DOI] [PubMed] [Google Scholar]
- Cheng H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. IV. Paneth cells. Am J Anat. 1974 Dec;141(4):521–535. doi: 10.1002/aja.1001410406. [DOI] [PubMed] [Google Scholar]
- Desai S. J., Mulherkar R., Wagle A. S., Deo M. G. Ontogeny of enhancing factor in mouse intestines and skin. Histochemistry. 1991;96(4):371–374. doi: 10.1007/BF00271359. [DOI] [PubMed] [Google Scholar]
- Dinsdale D., Biles B. Postnatal changes in the distribution and elemental composition of Paneth cells in normal and corticosteroid-treated rats. Cell Tissue Res. 1986;246(1):183–187. doi: 10.1007/BF00219016. [DOI] [PubMed] [Google Scholar]
- Gordon J. I., Schmidt G. H., Roth K. A. Studies of intestinal stem cells using normal, chimeric, and transgenic mice. FASEB J. 1992 Sep;6(12):3039–3050. doi: 10.1096/fasebj.6.12.1521737. [DOI] [PubMed] [Google Scholar]
- Granholm T., Fröysa B., Lundström C., Wahab A., Midtvedt T., Söder O. Cytokine responsiveness in germfree and conventional NMRI mice. Cytokine. 1992 Nov;4(6):545–550. doi: 10.1016/1043-4666(92)90017-l. [DOI] [PubMed] [Google Scholar]
- Huttner K. M., Selsted M. E., Ouellette A. J. Structure and diversity of the murine cryptdin gene family. Genomics. 1994 Feb;19(3):448–453. doi: 10.1006/geno.1994.1093. [DOI] [PubMed] [Google Scholar]
- Loeffler M., Birke A., Winton D., Potten C. Somatic mutation, monoclonality and stochastic models of stem cell organization in the intestinal crypt. J Theor Biol. 1993 Feb 21;160(4):471–491. doi: 10.1006/jtbi.1993.1031. [DOI] [PubMed] [Google Scholar]
- Markowitz A. J., Wu G. D., Birkenmeier E. H., Traber P. G. The human sucrase-isomaltase gene directs complex patterns of gene expression in transgenic mice. Am J Physiol. 1993 Sep;265(3 Pt 1):G526–G539. doi: 10.1152/ajpgi.1993.265.3.G526. [DOI] [PubMed] [Google Scholar]
- Mulherkar R., Rao R., Rao L., Patki V., Chauhan V. S., Deo M. G. Enhancing factor protein from mouse small intestines belongs to the phospholipase A2 family. FEBS Lett. 1993 Feb 15;317(3):263–266. doi: 10.1016/0014-5793(93)81289-c. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J., Greco R. M., James M., Frederick D., Naftilan J., Fallon J. T. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989 May;108(5):1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaberg L., Nexø E., Damsgaard Mikkelsen J., Seier Poulsen S. Immunohistochemical localisation and developmental aspects of epidermal growth factor in the rat. Histochemistry. 1988;89(4):351–356. doi: 10.1007/BF00500636. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Hertz J. M., Gordon J. I. Mapping enteroendocrine cell populations in transgenic mice reveals an unexpected degree of complexity in cellular differentiation within the gastrointestinal tract. J Cell Biol. 1990 May;110(5):1791–1801. doi: 10.1083/jcb.110.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. H., Winton D. J., Ponder B. A. Development of the pattern of cell renewal in the crypt-villus unit of chimaeric mouse small intestine. Development. 1988 Aug;103(4):785–790. doi: 10.1242/dev.103.4.785. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Miller S. I., Henschen A. H., Ouellette A. J. Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol. 1992 Aug;118(4):929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T. C., Roth K. A., Gordon J. I. Use of transgenic mice to map cis-acting elements in the liver fatty acid-binding protein gene (Fabpl) that regulate its cell lineage-specific, differentiation-dependent, and spatial patterns of expression in the gut epithelium and in the liver acinus. J Biol Chem. 1993 Aug 25;268(24):18345–18358. [PubMed] [Google Scholar]
- Whitehead R. H., VanEeden P. E., Noble M. D., Ataliotis P., Jat P. S. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sauvage F. J., Keshav S., Kuang W. J., Gillett N., Henzel W., Goeddel D. V. Precursor structure, expression, and tissue distribution of human guanylin. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9089–9093. doi: 10.1073/pnas.89.19.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]




