Abstract
Background
Traumatic brain injury (TBI) may be a risk factor for criminal behaviour however multiple factors potentially confound the association.
Methods
Record linkage and Cox proportional hazards regression analyses were used to examine the association between hospital-recorded TBI (n = 7,694) and subsequent first criminal conviction in a retrospective cohort matched 1:3 with 22,905 unaffected community controls and full-sibling controls (n = 2,397). Aboriginality, substance abuse, social disadvantage, and mental illness were included in analyses as potential confounders
Results
In multivariable models, relative to general population controls, TBI was associated with any conviction (males: Hazard Ratio (HR) = 1·58 (95% CI 1·46 to 1·72); females: HR = 1·52 (95% CI 1·28 to 1·81)); and similar Hazard Ratios were obtained for the sibling analyses in males (HR = 1.68 (95% CI 1.31-2.18)) and females (HR 1.27 (95% CI 0.71-2.29)). TBI was also associated with violent convictions relative to the general population, (males: HR = 1.65 (95% CI 1.42 to 1.92); females HR = 1.73 (95% CI 1.21 to 2.47)), and in analyses with sibling controls in men (HR = 1.89 (95% CI 1.20-3.00)), but not in women (HR 0.73, 95% CI 0.29-1.81)).
Conclusion
The results support a modest causal link between TBI and criminality after comprehensive adjustment for confounding. Reducing the rate of TBI, a major public health imperative, might have benefits in terms of crime reduction.
Introduction
Studies of offender populations consistently show high rates of past traumatic brain injury (TBI) [1] and some investigators suggest that many offences may be a consequence of TBI-related behavioural dysregulation [2]. The societal, public health, criminological, and custodial implications of an established causal association between TBI and subsequent offending would be profound if, as the investigators of one study proposed, ‘a head injury leads victims to participate in more than half of the crimes that come to the attention of police and that result in incarceration’ [2]. With increasing concerns expressed about TBI among soldiers returning from conflicts such as Afghanistan and in those engaged in body contact sports a rigorous examination of the purported link between TBI and criminality is relevant, timely and important [3–4].
Most previous studies bearing on this issue have been cross-sectional [1–2, 5] but two large longitudinal studies using record linkage have also been published. In one, TBI in childhood was associated with a 1·7-fold increased risk of criminal conviction later in life relative to general population controls [6]. In the other, TBI leading to hospitalisation was associated with a 3-fold increased risk of a conviction for a violent offence relative to general population controls and a two-fold increased risk relative to non-exposed siblings [7]. More recently, results from a longitudinal birth cohort of children born in Christchurch New Zealand, indicated an association between TBI and self-reported arrests, property offenses and violent offenses [8]. For the current longitudinal retrospective cohort study, we use linked data and account for important confounders not comprehensively examined in these previous studies to obtain unbiased estimates of the association between a hospital-documented TBI and first criminal conviction, including a conviction for violence specifically [9–10].
Methods
Study sample
We used whole-population data linkage to identify individuals born in Western Australia (WA) between 1980–1985 inclusive who had attended a hospital in WA with record-evidence of a TBI. For each such individual, we randomly selected three individuals of the same sex and year of birth born in WA who had never been admitted to hospital for TBI (‘population controls’). We also identified all same-sex full-siblings without record-evidence of TBI born within +/- 5 years of the TBI-affected proband to serve as controls. When more than one suitable sibling was identified, the one nearest in age to their exposed counterpart was selected for the analyses.
Evidence of TBI
Evidence of TBI was defined as a hospital admission with a primary admitting or additional diagnosis for any of the TBI diagnostic codes (S1 Table) [11–12].
Other risk factors
Proxies for potential confounders including social disadvantage, mental illness, Aboriginality, and substance abuse were obtained as follows. A measure of socioeconomic disadvantage was derived from the Index of Relative Social Disadvantage from the Australian Bureau of Statistics (ABS) [13], ascertained at a postcode level for usual residence at the time of birth. Aboriginal descent was defined by the presence of at least one record across all available datasets identifying the person as Aboriginal [14]. Ever had a mental health problem was defined as having a mental health-related hospital admission or public outpatient mental health service contact according to ICD diagnostic codes: ICD-10 ‘F00’-‘F99’ or ICD-9 ‘290’-‘319’. Presence of a drug record was defined as having received a drug- or alcohol-related treatment episode recorded by the WA Drug and Alcohol Office.
Definition of outcome
The outcome of interest was an incident criminal conviction as determined by WA Department of Corrective Service’s records, including juvenile offending (up to 2009) and adult offending up to September 2011 resulting in a criminal conviction by the WA Court system. This included community-based orders and custodial sentences. Additional analyses examined convictions for violent offences only, comprising homicide, assault (including sexual assault), robbery, or murder. In Australia, criminal responsibility begins from the age of 10 and we therefore excluded anyone who died before reaching this age. Participants with a criminal conviction prior to their first-time TBI were excluded from the study.
Datasets
We used de-identified whole-population administrative data linked across the WA Departments of Health and Corrective Services, and the WA Drug and Alcohol Office. Data from the ABS were used to assign geocodes to postcodes provided in midwives’ notifications data. The State Health datasets included all midwives’ notifications, hospital separation records, death registrations, public outpatient and inpatient mental health records, and drug and alcohol treatment records. For our study, the midwives’ notification data contained information for all registered births in WA from 1980–1985, and for siblings until 1990. This information was used to identify each individual’s year and month of birth and sex. The hospital morbidity data contained information on all public and private hospital separations (discharges or deaths) in WA with corresponding diagnostic and procedural information coded according the ICD coding system from 1980–2010 [11–12]. Data from the WA Mortality Register were extracted for all deaths in WA from 1980–2011 for the study sample. Mental health data included information on mental health inpatient and publicly-funded outpatient admissions from 1980–2010. The WA Drug and Alcohol Office included information for all drug and alcohol treatment episodes recorded by the Office from 1980–2006. The WA Corrective Services datasets included juvenile (1993–2009) and adult (1997–2011) records as well as data on community corrections orders (i.e. non-custodial sentences). The Australian Census data contain information on neighbourhood economic disadvantage. All datasets were linked by the WA Data Linkage Branch at the WA Department of Health using established best-practice probabilistic matching protocols [15–16]. No other source of medical or judicial information was available to us; the datasets used were comprehensive with minimal missing data.
Statistical Analysis
We included only individuals with their first conviction after the first diagnosis of TBI, and those who survived a full year from their first TBI to allow adequate time for an outcome to occur. To ensure consistency of risk periods for TBI-exposed and unexposed groups, controls were assigned a pseudo-TBI date based on the age at which the TBI occurred in the matched exposed cohort member and only controls without a conviction prior to that assigned date and who survived until the age of 10 and for 12 months following their assigned TBI date were included. All individuals were followed from their TBI date (pseudo or actual) until their first conviction, death, or 31st December 2006, whichever occurred first. Males and females were analysed separately. Additional analysis looking at violent convictions only followed individuals until their first conviction for a violent offence, death, or 31st December 2006.
Aboriginal descent, disadvantaged background, mental illness, treatment for substance abuse disorders and conviction records were compared between TBI and controls using the Chi-square test.
Univariable and multivariable Cox proportional hazard regression models were used to assess the association between TBI and risk of criminal conviction. Covariates including treatment for drug or alcohol abuse, mental illness, Aboriginality, socioeconomic disadvantage and year of birth were included in adjusted analysis.
The proportionality assumptions of the hazard functions were assessed for individual covariates and globally using Schoenfeld´s residuals [17]. At the global level, the proportionality assumption was not violated in any of the models considered (p-values in multivariate models were ranged from 0.1115–0.1725); however at the individual level, the proportionality assumption was violated for indigenous status in females (p-value = 0.0364). Following published guidelines [18], we stratified our analysis on the non-proportional predictor, indigenous status, and obtained estimates within each stratum indicating overall goodness of the fit of the final model. The final model fitted the data reasonable well except for the adjusted logistic regression in twins (males and females); and results for these analyses were excluded as the models did not converge. Proportionality assumptions on the hazards functions were also assessed for all the other subgroup analyses; data supported the proportionality assumptions on the hazard ratios: goodness of fit of the final models were at acceptable levels. Further evaluation using the Cox-Snell residuals provided additional reassurance that the models adopted fitted the data satisfactorily [18].
For analyses of siblings/twins analysis, univariable and multivariable stratified Cox regression models were used with separate baseline hazard functions across the families [17]. We undertook separate analyses for siblings and twins. Data were analysed using SAS Version 9·3 (SAS Institute Inc., Cary, NC, USA) and STATA 10·0 (College Station, TX, USA) using a two-tailed 5% significance level. Since Aboriginal descent, disadvantaged background, mental illness and substance abuse disorders have been associated both with conviction rates and TBI [1–2, 5–10], and are also potential effects modifiers, we included proxies for these factors as covariates and assessed their interactions with TBI, and also adjusted for year of birth.
Ethics Statement
The protocol was approved by the Department of Health WA, Western Australian Aboriginal Health Ethics Committee (WAAHEC), Curtin University, and the WA Department of Corrective Services Research and Evaluation Committee. The data collected was de-identified and was therefore analysed anonymously.
Results
Of the total 136 100 individuals born from 1980–1985, 8242 were initially identified as having sustained a TBI resulting in a hospital admission in WA from 1980–2006. We excluded 469 convicted before the date of their TBI, 24 who died before the age of 10 years, and 55 who died within one year of their incident TBI (94% of whom died within two months of their TBI) leaving 7694 individuals in the cohort (5018 males, 2676 females,) (Fig 1). The median age at first TBI was 10·6 years in males (inter quartile range [IQR] 4·6–16·2) and 6·9 years in females (IQR 2·8–14·1). The medial follow-up time was 12·5 years for males (IQR 6·8–18·6) and 16·6 years for females (IQR 9·4–20·8).
Fig 1. Overview of Sampling.
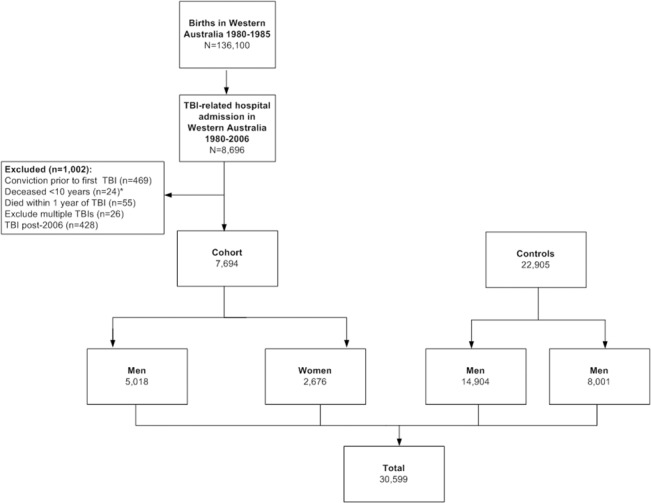
Flowchart depicts the participant numbers that were included and excluded. *10 years is considered the age of criminal responsibility in Australia.
Characteristics of the study groups are shown in S2 and S3 Tables. Relative to non-exposed community controls, those with a TBI had an approximately two fold rate of a conviction record (males: 18% vs 10%, p<0·0001; females: 9% vs 4%, p <0·0001), mental illness (males:19% vs 9%, p<0·001; females:22% vs 13%, p<0·001), and Aboriginal descent (males: 11% vs 6%, p<0·0001; females: 17% vs 6%, p<0·001).
Compared with community controls in unadjusted analyses TBI increased the risk of any conviction in males (Hazard Ratio (HR) = 1·85 (95% confidence interval (CI) 1·70 to 2·01)), and females (HR = 2·20 (95% CI 1·86 to 2·59)) (Table 1).
Table 1. Adjusted Hazard Ratios (95% CI) of Males (n = 19,992) and Females (n = 10,677) having a “Corrective Services Record” (CSR).
| Groups | Sub-Groups | Overall [n(%)] | CSR [n(%)] | Adjusted Hazard Ratio (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | ||
| Traumatic Brain Injury | Not exposed | 14904 (75) | 8001 (75) | 1554 (10) | 332 (4) | 1 | 1 | ||
| Exposed | 5018 (25) | 2676 (25) | 920 (18) | 238 (9) | 1·58 (1·46 to 1·72) | 1·52 (1·28 to 1·81) | <0·001 | <0·001 | |
| Drug and alcohol treatment record 1 | No | 19844 (99) | 10628 (99) | 2457 (12) | 556 (5) | 1 | 1 | ||
| Yes | 79 (0·4) | 49 (0·46) | 17 (22) | 14 (29) | 1·96 (1·21 to 3·17) | 5·62 (3·27 to 9·67) | 0·006 | <0·001 | |
| Mental illness diagnosis 1 | No | 17611 (88) | 9056 (85) | 2146 (12) | 448 (5) | 1 | 1 | ||
| Yes | 2311 (12) | 1621 (15) | 328 (14) | 122 (8) | 1·10 (0·97 to 1·23) | 1·23 (1·01 to 1·52) | 0·145 | <0·001 | |
| Indigenous status | No | 18508 (93) | 9705 (91) | 1780 (10) | 330 (3) | 1 | 1 | ||
| Yes | 1414 (7) | 972 (9) | 694 (49) | 240 (25) | 4·98 (4·53 to 5·48) | 6·52 (5·41 to 7·85) | <0·001 | <0·001 | |
| Index of Disadvantage 2 | Lowest | 4801 (24) | 2588 (24) | 421 (9) | 80 (3) | 1 | 1 | ||
| Low | 4832 (24) | 2574 (24) | 489 (10) | 124 (5) | 1·01 (0·89 to 1·15) | 1·31 (0·98 to 1·73) | 0·848 | 0·065 | |
| High | 4921 (25) | 2626 (25) | 610 (12) | 118 (4) | 1·16 (1·02 to 1·31) | 1·08 (0·81 to 1·43) | 0·024 | 0·620 | |
| Highest | 4678 (24) | 2509 (24) | 885 (19) | 233 (9) | 1·42 (1·26 to 1·61) | 1·54 (1·17 to 2·01) | <0·001 | 0·002 | |
| Missing | 690 (4) | 380 (4) | 69 (10) | 15 (4) | 0·85 (0·65 to 1·10) | 1·03 (0·59 to 1·78) | 0·197 | 0·926 | |
| Year of Birth | - | - | - | - | - | 1·03 (1·01 to 1·05) | 1·01 (0·96 to 1·06) | 0·015 | 0·619 |
1 counted as “0” if occurred after the conviction
2 Highest index of disadvantage represents the lowest level of socioeconomic status (SES)
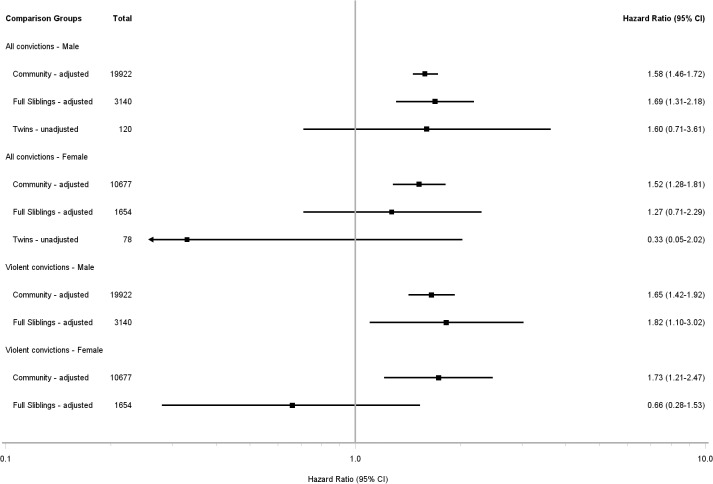
In multivariable analyses there were no significant interactions between TBI and other risk factors for conviction and, compared with community controls, TBI was associated with increased risk for all offending in males (HR = 1·58 (95% CI 1·46 to 1·72)) and females (HR = 1·73 (95% CI 1·21 to 2·47)).When same-sex full-sibling controls were used in the adjusted analyses, increased risk of offending was evident among TBI-exposed males (HR = 1·69 (95% CI 1·31–2.18)) but was not significant in females (HR = 1·27 (95% CI 0·71 to 2.29)). In twins discordant for TBI exposure, models adjusted for covariates did not converge but in unadjusted analyses, males (HR = 1.60, (95% CI0.71–3.61)) but not females (HR = 0.33 (95% CI 0.05–2.02)) had a positive HR, in neither case statistically significant (Fig 2). For both sexes, increased hazard of offending was associated with a mental health record, drug and alcohol record, Aboriginality, and increased social disadvantage.
Fig 2. Forest Plot of Unadjusted and Adjusted Hazard Ratios for Any and Violent Convictions.
Figure depicting the associations between TBI and any violent offending, relative to the non- exposed comparison groups: general community, full siblings, or twins.
For violent convictions, relative to the general community, TBI was also associated with increased risk in men (HR = 1·65 (95% CI 1·42 to 1·92)) and women (HR = 1.73 (95% CI 1.21 to 2.47)) (Table 2). Among full siblings discordant for TBI, TBI was associated with violent offending in men (HR = 1.82 (95% CI 1.10–3.020)) but not in women (HR = 0.66 (95% CI 0.28–1.53)). There were insufficient data to conduct meaningful analyses with respect to violent convictions amongst twins for either sex (S4 Table).
Table 2. Adjusted Hazard Ratios (95% CI) of Males (n = 19,992) and Females (n = 10,677) having a “Violent Offence Record” (VOR).
| Groups | Sub-Groups | Overall [n(%)] | VOR [n(%)] | Adjusted Hazard Ratio (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | Males | Females | ||
| Traumatic Brain Injury | Not exposed | 14904 (75) | 8001 (75) | 404 (3) | 64 (0.80) | 1 | 1 | ||
| Exposed | 5018 (25) | 2676 (25) | 303 (6) | 66 (2) | 1·65 (1·42 to 1·92) | 1·73 (1·21 to 2·47) | <0·001 | 0·002 | |
| Drug and alcohol treatment record 1 | No | 19844 (99) | 10628 (99) | 702 (4) | 126 (1) | 1 | 1 | ||
| Yes | 78 (0·4) | 49 (0·46) | 5 (6) | 4 (8) | 1·79 (0·74 to 4·35) | 6·55 (2·34 to 18·30) | 0·197 | <0·001 | |
| Mental illness diagnosis 1 | No | 17611 (88) | 9056 (85) | 591 (3) | 96 (1) | 1 | 1 | ||
| Yes | 2311 (12) | 1621 (15) | 116 (5) | 34 (2) | 1·37 (1·12 to 1·68) | 1·44 (0·96 to 2·17) | 0·002 | 0·076 | |
| Indigenous status | No | 18508 (93) | 9705 (91) | 360 (2) | 46 (0·47) | 1 | 1 | ||
| Yes | 1414 (7) | 972 (9) | 347 (25) | 84 (9) | 10·25 (8·73 to 12·04) | 15·55 (10·48 to 23·07) | <0·001 | <0·001 | |
| Index of Disadvantage 2 | Lowest | 4801 (24) | 2588 (24) | 68 (1) | 11 (0·43) | 1 | 1 | ||
| Low | 4832 (24) | 2574 (24) | 133 (3) | 25 (9·97) | 1·48 (1·10 to 1·99) | 1·53 (0·75 to 3·14) | 0·009 | 0·243 | |
| High | 4921 (25) | 2626 (25) | 185 (4) | 36 (1) | 1·71 (1·29 to 2·28) | 1·79 (0·90 to 3·56) | <0·001 | 0·098 | |
| Highest | 4678 (24) | 2509 (24) | 305 (7) | 55 (2) | 1·94 (1·47 to 2·56) | 1·51 (0·76 to 3·00) | <0·001 | 0·234 | |
| Missing | 690 (4) | 380 (4) | 16 (2) | 3 (0·79) | 0·94 (0·54 to 1·63) | 1·19 (0·33 to 4·29) | 0·832 | 0·789 | |
| Year of Birth | - | - | - | - | - | 1·07 (1·02 to 1·12) | 1·00 (0·90 to 1·11) | 0·003 | 0·949 |
1 counted as “0” if occurred after the conviction
2 Highest index of disadvantage represents the lowest level of socioeconomic status (SES)
Discussion
The results of this retrospective cohort data linkage study are consistent with a modest increase in the risk of offending, including violent offending, following a hospital- documented TBI. The association was found in models adjusted for several important confounders and among siblings discordant for TBI and would be consistent with a causal role for TBI in the subsequent committal of offences.
Previous work suggests a range of possible mechanisms for the well-established association between TBI and offending. First, a TBI might, via sequelae such as behavioural dysregulation, aggression, or impulsivity, be causally implicated in offending behaviour [2, 7]. Our own previous studies suggest that the majority of TBIs reported by convicted offenders are mild, albeit often experienced on multiple occasions. While mild TBI rarely leads to significant cognitive or behavioural changes [19–20], mild TBI warranting admission to hospital has been associated with the subsequent development of adverse behaviours[8]. Results from the current study are consistent with such a mechanism.
Second, a TBI might be an independent consequence of a behavioural phenotype that also leads to offending [21]. Certain behaviours in early life that are associated with an increased risk of criminal behaviour in adulthood [22–23] also elevate the risk of head injury [24–25]. In the Dunedin Multidisciplinary Health and Development Study, behavioural ratings were made on children at the age of three. Compared with three year olds who were ‘well-adjusted’, those who were ‘under-controlled’ were, at the age of 21 y, more likely to be diagnosed with antisocial personality disorder, be recidivistic offenders, or have been convicted for a violent offence [22]. In a Finnish study, aggression in the form of bullying at age 8 independently predicted later violent, property and traffic offences [23]. Aggression in early life has also been associated with increased rates of subsequent head trauma and other injuries in childhood and adolescence [24–25]. Heritability of impulsivity, violence, and criminality has been well established in the literature [26–27]. Environmental exposures and genetic similarity is greatest in same sex twins—an ideal comparison group to take account of these potential confounds. In the current study, when siblings served as controls, the estimates of risk of offending associated with TBI were similar to those obtained using population controls, except for sub-analyses in women with small sample sizes or sparse outcomes (twins, violent offending). Within the recognised limitations of family studies (see below), results of the current study provide little support for mechanism two as a major explanation for the association of TBI and offending. Third, a TBI might lead to (or follow from) mental illness [28–29] which for complex and poorly-understood reasons is strongly associated with offending [30]. We repeated the analyses, recoding to include only instances of mental illness recorded prior to the occurrence of TBI (or pseudo TBI). The risk estimates of offending associated with TBI in these supplementary analyses were unchanged, suggesting that mental illness does not mediate risk associated with TBI in a causal pathway to offending. Fourth, a TBI could occur as a consequence of an alcohol/substance use disorder that is the proximate cause for offending [2, 5, 10]. Fifth, criminal activity or incarceration could lead to a TBI, an instance of reverse causation. Prisons are comparatively violent places. This seems unlikely to account for any but a small proportion of the excess TBI prevalence in offender populations, based on our own studies [20, 31]. Finally, other combinations or interactions between mechanisms might occur, e.g. following a TBI, alcohol abuse may have greater behavioural consequences, perhaps leading to offending behaviour.
The results from our analyses using population controls are similar to those of Timonen et al., whose unadjusted risk-estimate for all convictions associated with a TBI was 1·7 [6]. The lack of sibling controls renders their estimate of risk less compelling, in terms of a causal association between TBI and criminality. In relation to violent convictions, the risk estimates related to TBI that we obtained were more modest than those reported by Fazel et al., [7] but there were some important methodological differences that might account for the discrepancies. In the Fazel study, individuals were not excluded from the analyses if they had been convicted of a crime before the occurrence of TBI, although only convictions after the TBI were ‘counted’. This is important because previous criminality may be more prevalent among individuals who sustain a TBI and past criminality is a risk factor for subsequent crime [32]. Interestingly, in a study of US Veterans post deployment, those screen positive for a TBI were no more likely to have a history of subsequent arrest than those screen negative, after adjustment for relevant confounders including previous criminal arrests [33].
In the present study, we excluded from the analyses all individuals with criminal convictions prior to their TBI (with parallel exclusions in the controls), an appropriate strategy, we believe, for evaluating the hypothesis that TBI ‘causes’ criminal behaviour. Also by contrast with the Fazel study, we adjusted for mental illness as a potential confound and conducted additional analyses using same sex twin controls. The study by McKinlay et al. included offending data derived from self-report, psychiatric history was not included as a covariate in the analyses, nor were siblings available as controls although other family factors were considered [8].
Our study had some limitations. Conviction records provide an imprecise measure of offending behaviour, given the evidence to suggest that much offending goes undetected and in this respect the McKinlay study has advantages relative to our own [34, 8]. Hospital admission records provide incomplete ascertainment of TBIs, and TBIs in the comparison group, albeit mild, could potentially bias the results toward the null. Despite certain strengths—particularly relating to control for genetic factors—sibling/twin studies have the potential for bias introduced by measurement error or unmeasured confounding, especially for analyses that depend on discordant pair analyses such as in our study [35–36]. For example, substance abusers, at increased risk both for offending and TBI, may be less inclined to seek hospital treatment for mild TBI than non-abusers (i.e. selective under-ascertainment of the exposure), a phenomenon that could bias toward the null and may have contributed to the somewhat unexpected findings in several of the sub analyses in women [37]. Indeed, another study limitation is that our proxy for drug and alcohol abuse did not capture levels of exposure that would be expected and possibly reflect the lack of treatment opportunities in this population. Finally, our results relate to time to first criminal conviction, and TBI as a single binary exposure without stratification by severity, although all those with TBI had been injured sufficiently to warrant hospitalization. Strengths of the study include the cohort size, stratification by sex, inclusion of multiple control groups, including twins of the probands, and our examination of the impact of comorbidity, especially mental illness, on the risk of a conviction.
In conclusion, the results from the current study would be consistent with a causal relationship between TBI and subsequent criminal convictions, and convictions for violence in particular, in both sexes. Successful reduction in the prevalence of TBI, a major public health imperative, could also have benefits in terms of crime rate reduction. Future research directions will include examining the relative contribution of specific factors implicated in the association between TBI and offending, and the development of predictive models that may help to better explain this complex relationship. Gender and ethnic differences, maternal and birth characteristics, and age at TBI may be important in this regard.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank an anonymous statistical reviewer whose insightful comments contributed significantly to the final formulation of this work.
We thank Tammie Moore for her assistance with manuscript preparation.
The results of this research should not be considered as either endorsed by the Western Australian Department of Corrective Services (DCS) or an expression of the policies or view of the DCS.
EM wishes to acknowledge support from the Healy Medical Research Foundation.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding was supplied by the National Health and Medical Research Council (grant # 533504) to TB, PWS, RT, DBP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Shiroma EJ, Ferguson PL, Pickelsimer EE (2010) Prevalence of traumatic brain injury in an offender population: A Meta-Analysis. J Correct Health Care 16(2):147–159. 10.1177/1078345809356538 [DOI] [PubMed] [Google Scholar]
- 2. Sarapata M, Herrman D, Johnson T, Aycock R (1998) The role of head injury in cognitive functioning, emotional adjustment and criminal behaviour. Brain Inj 12(10):821–842. [DOI] [PubMed] [Google Scholar]
- 3. Taylor BC, Hagel EM, Carlson KF, Cifu DX, Cutting A, Bidelspach DE, et al. (2012) Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med Care 50:342–6. 10.1097/MLR.0b013e318245a558 [DOI] [PubMed] [Google Scholar]
- 4. Langlois JA, Rutland-Brown W, Wald MM (2006) The Epidemiology and Impact of Traumatic Brain Injury: A Brief Overview. J Head Trauma Rehab 21: 375–378. [DOI] [PubMed] [Google Scholar]
- 5. Schofield PW, Butler T, Hollis S, Smith N, Lee S, Kelso WM (2006) Traumatic brain injury among Australian prisoners: rates, recurrence and sequelae. Brain Inj 20(5): 499–506. [DOI] [PubMed] [Google Scholar]
- 6. Timonen M, Miettunen J, Hakko H, Zitting P, Veijola J, von Wendt L, et al. (2002) The association of preceding traumatic brain injury with mental disorders, alcoholism and criminality: the Northern Finland 1966 birth cohort study. Psychiat Res 113(3): 217–226. [DOI] [PubMed] [Google Scholar]
- 7. Fazel S, Lichtenstein P, Grann M, Långström N (2001) Risk of violent crime in individuals with epilepsy and traumatic brain injury: A 35-year Swedish population study. PLoS Med 8(12): e1001150 10.1371/journal.pmed.1001150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McKinlay A, Corrigan J, Horwood LJ, Fergusson DM (2013) Substance abuse and criminal activities following traumatic brain injury in childhood adolescence and early adulthood. J Head Trauma Rehabil 10.1097/HTR.0000000000000001 [Published ahead of print] [DOI] [PubMed] [Google Scholar]
- 9. Jamieson LM, Harrison JE, Berry JG (2008) Hospitalisation for head injury due to assault among Indigenous and non-Indigenous Australians, July 1999—June 2005. Med J Australia 188(10): 576–579. [DOI] [PubMed] [Google Scholar]
- 10. Walker R, Hiller M, Staton M, Leukefeld CG (2003) Head injury among drug abusers: An indicator of co-occurring problems. J Psychoactive Drugs. 35(3): 343–353. [DOI] [PubMed] [Google Scholar]
- 11.The Australian Version of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). (Second Edition) Volume 1, 1 July 1996, National Coding Centre, Faculty of Health Sciences, The University of Sydney, NSW 2141, Australia.
- 12.The International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification (ICD-10-AM)–Tabular List of Diseases and Alphabetic Index of Diseases, (Seventh Edition) 2010, Commonwealth of Australia, National Centre for Classification in Health, Faculty of Health Sciences, The University of Sydney, NSW 1825, Australia.
- 13.Australian Bureau of Statistics (2006) Socioeconomic Indexes for Areas (SEIFA)-Technical paper. cat. no. 2039.0.55.001. ABS, Canberra, ACT, Australia.
- 14. Preen D, Holman CDJ, Lawrence DM, Baynham NJ, Semmens JB (2003) Accuracy of recorded comorbidity with medical record linkage: a hospital and primary care validation study. Australas Epidemiol 10(3): 24–25. [Google Scholar]
- 15. Kelman CW, Bass AJ, Holman CDJ (2002) Research use of linked health data—a best practice protocol. Aust N Z J Public Health 26(3): 251–255. [DOI] [PubMed] [Google Scholar]
- 16. Briffa TG, Sanfilippo FM, Hobbs MST, Ridout SC, Katzenellenbogen JM, Thompson PL, et al. (2010) Under-ascertainment of Aboriginality in records of cardiovascular disease in hospital morbidity and mortality data in Western Australia: A record linkage study. BMC Med Res Methodol 10(111): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hosmer DW Jr, Lemeshow S (1999) Applied survival analysis: regression modelling of time to event data. New York: John Wiley. [Google Scholar]
- 18.Institute for Digital Research and Education. Introduction to SAS. UCLA: Statistical Consulting Group. Available: http://www.ats.ucla.edu/stat/sas/notes2/. Accessed 2015 Apr 24.
- 19. Holm L, Cassidy JD, Carroll IJ, Borg J (2005) Summary of the WHO collaborating centre for neurotrauma task force on mild traumatic brain injury. J Rehabil Med 37(3): 137–141. [DOI] [PubMed] [Google Scholar]
- 20. Schofield PW, Butler T, Hollis S, D’Este C (2011) Are prisoners reliable survey respondents? A validation of self-reported traumatic brain injury (TBI) against hospital medical records. Brain Inj 25(1): 74–82. 10.3109/02699052.2010.531690 [DOI] [PubMed] [Google Scholar]
- 21. Turkstra L, Jones D, Toler L (2003) Brain injury and violent crime. Brain Inj 17(1): 39–47. [DOI] [PubMed] [Google Scholar]
- 22. Caspi A, Moffitt TE, Newman DL, Silva PA (1996) Behavioral observations at age 3 years predict adult psychiatric disorders: longitudinal evidence from a birth cohort. Arch Gen Psychiatry 53(11): 1033–1039. [DOI] [PubMed] [Google Scholar]
- 23. Sourander A, Klomek AB, Kumpulainen K, Puustjärvi A, Elonheimo H, Ristkarei T, et al. (2011) Bullying at age eight and criminality in adulthood: findings from the Finnish Nationwide 1981 Birth Cohort Study. Soc Psychiatry Psychiatr Epidemiol 46(12): 1211–1219. 10.1007/s00127-010-0292-1 [DOI] [PubMed] [Google Scholar]
- 24. Bijur PE, Haslum M, Golding J (1990) Cognitive and behavioral sequelae of mild head injury in children. Pediatrics 86(3): 337–344. [PubMed] [Google Scholar]
- 25. Cobb BK, Cairns BD, Miles MS, Cairns RB (1995) A longitudinal study of the role of sociodemographic factors and childhood aggression on adolescent injury and ‘close calls’. J Adolesc Health 17(6): 381–388. [DOI] [PubMed] [Google Scholar]
- 26. Frisell T, Pawitan Y, Långström N, Lichtenstein P (2012) Heritability, assertive mating and gender differences in violent crime: results from a total population sample using twin, adoption and sibling models. Behav Genet 42: 3–18. 10.1007/s10519-011-9483-0 [DOI] [PubMed] [Google Scholar]
- 27. Rowe DC, Farrington DP (1997) The familial transmission of criminal convictions. Criminology 35(1): 177–201. [Google Scholar]
- 28. Fann JR, Burington B, Leonetti A, Jaffe K, Katon WJ, Thompson RS (2004) Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Arch Gen Psychiatry 61(1): 53–61. [DOI] [PubMed] [Google Scholar]
- 29. Fann JR, Leonetti A, Jaffe K, Katon WJ, Cummings P, Thompson RS (2002) Psychiatric illness and subsequent traumatic brain injury: A case control study. J Neurol Neurosurg Psychiatry 72(5): 615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hodgins S, Mednick SA, Brennan PA, Schulsinger F, Engberg M (1996) Mental disorder and crime: evidence from a Danish birth cohort. Arch Gen Psychiatry 53(6): 489–496. [DOI] [PubMed] [Google Scholar]
- 31. Schofield PW, Butler G, Hollis SJ, Smith NE, Lee SJ, Kelso WM (2006) Neuropsychiatric correlates of traumatic brain injury (TBI) among Australian prison entrants. Brain Inj 20(13–14): 1409–1418. [DOI] [PubMed] [Google Scholar]
- 32. Mulder E, Brand E, Bullens R, van Marle H (2011) Risk Factors for Overall Recidivism and Severity of Recidivism in Serious Juvenile Offenders. Int J Offender Ther Comp Criminol 55: 118 10.1177/0306624X09356683 [DOI] [PubMed] [Google Scholar]
- 33. Elbogen EB, Johnson SC, Newton VM, Straits-Troster K, Vasterling JJ, Wagner H, et al. (2012) Criminal justice involvement, trauma and negative affect in Iraq and Afghanistan war era veterans. J Consult Clin Psychol 80(6): 1097–1102. 10.1037/a0029967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mayhew P (2003) Counting the cost of crime in Australia. Trends Iss Crime Crim Justice 247: 1–8. [Google Scholar]
- 35. Lahey BB, D’Onofrio BM (2010) All in the family: Comparing siblings to test causal hypotheses regarding environmental influences on behavior. Curr Direc Psychol Sci. 19(5):319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frisell T, Öberg S, Kuja-Halkola R, Sjölander A (2012) Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology 23(5):713–20. 10.1097/EDE.0b013e31825fa230 [DOI] [PubMed] [Google Scholar]
- 37. Dams-O’Connor K, Cantor JB, Brown M, Dijkers MP, Spielman LA, Gordon WA (2014) Screening for Traumatic Brain Injury: Findings and Public Health Implications. J Head Trauma Rehabil 29(6):479–489. 10.1097/HTR.0000000000000099 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.