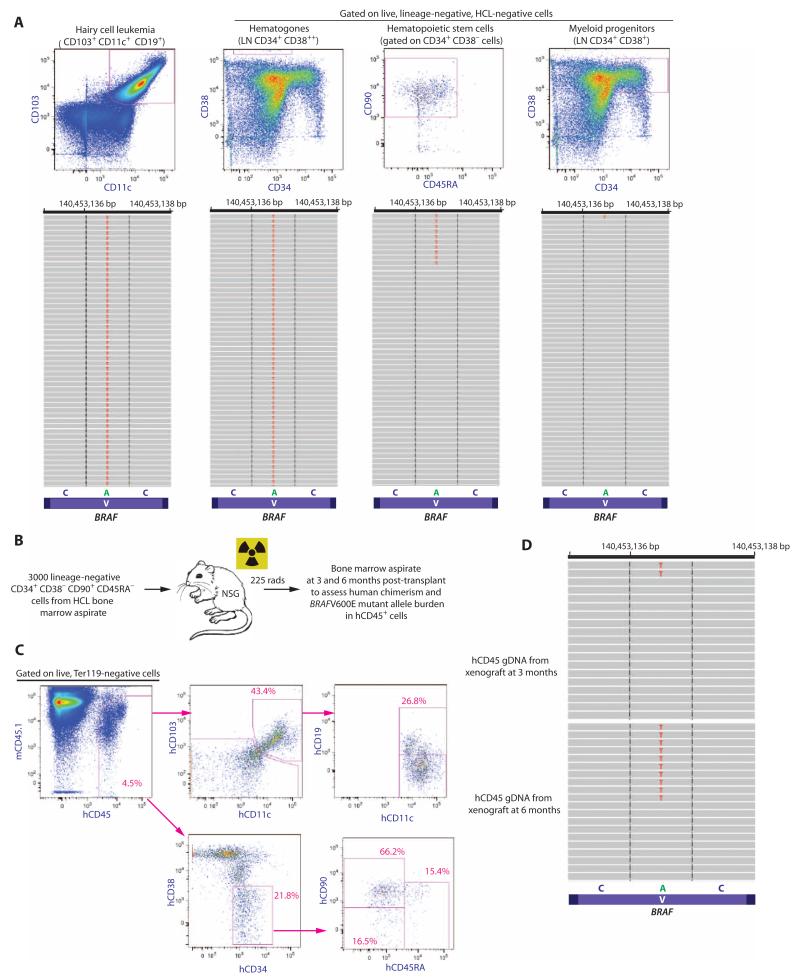
Fig. 2. Quantitative analysis of the BRAFV600E mutation in HSCs from HCL patients and functional self-renewal capacity of BRAFV600E-mutant HSCs.
(A) Representative FACS analysis and quantitative sequencing analysis revealing the VAF of the BRAF c.T1860A p.V600E mutation in HCL cells, hematogones, HSCs, and MP cells from an HCL patient (for clarity, only 52 reads are displayed). cDNA from double FACS-sorted cell populations were used for MiSeq targeted sequencing. (B) Schema of xenograft experiment where 3000 HSCs from a BRAFV600E-mutant HCL patient were injected into sublethally irradiated NSG mice followed by flow cytometric analysis of human engraftment and HCL cells, as well as quantification of the BRAFV600E mutation by sequencing analysis. (C) At 6 months after transplant, overall human chimerism was 4.5% with the presence of human HSCs (hCD45+ hCD34+ hCD38− hCD90+ hCD45RA−) and a cell population with the immunophenotype of HCL cells (hCD45+ hCD103+ hCD19+ hCD11c+). (D) MiSeq sequencing analysis at 100× coverage reveals BRAFV600E mutation in 4 and 9% of hCD45+ cell genomic DNA at 3 and 6 months, respectively.