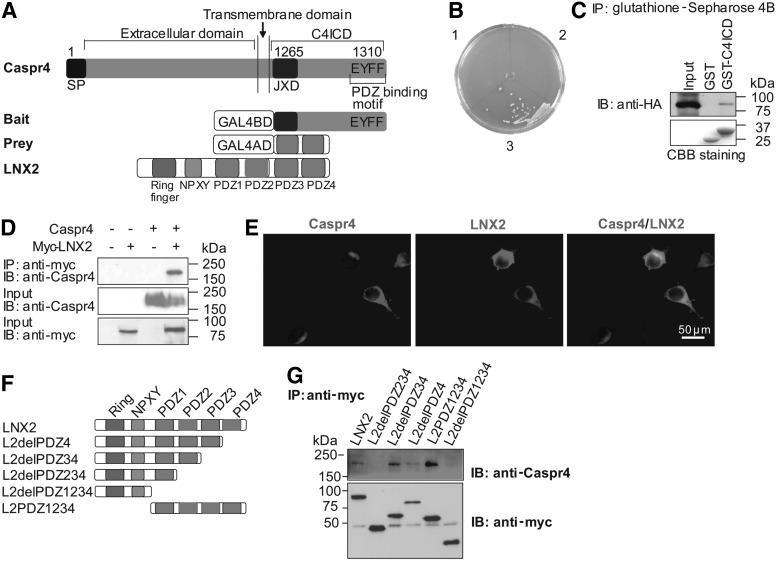
FIG. 3.
Ligand of Numb protein X2 (LNX2) interacts with Caspr4. (A) Schematic representations of full-length Caspr4, the bait protein that has the last 47 amino acids of the Caspr4 cytoplasmic domain (C4ICD, amino acids 1,265–1,310), the prey clone that codes the amino acids 433–690 of LNX2, and full-length LNX2 that contains one Ring finger, one NPXY, and four PDZ domains. (B) Yeast cells were transformed with the following plasmids: Caspr4ICD/pGBKT7 and pGADT7 (1), pGBKT7 and LNX2/pGADT7 (2), and Caspr4ICD/pGBKT7 and LNX2/pGADT7 (3). Transformants were spread onto SD/ −Ade/ −His/ −Leu/ −Trp medium plate containing X-α-Gal. Colonies were only observed in yeast cells coexpressing C4ICD and LNX2. (C) Protein extracts from LNX2-HA-transfected COS7 cells were incubated and pulled-down with GST and GST-C4ICD proteins. Bound proteins were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with anti-HA antibody (upper panel) or stained with Coomassie brilliant blue (CBB staining; lower panel). (D) Caspr4 was cotransfected with LNX2-myc into HEK293T cells. Immunoprecipitation was performed with an anti-myc antibody and probed with an anti-Caspr4 antibody. The inputs were immunoblotted with both antibodies against Caspr4 and myc as indicated. (E) HEK293T cells were cotransfected with Caspr4 and LNX2-myc. After 24 h post-transfection, double immunofluorescence staining was performed on these cells by using antibodies against Caspr4 and myc. Scale bar: 50 μm. (F, G) Schematic representation of truncated LNX2 constructs, which were tagged with a myc at their N-terminus (F). Caspr4 was cotransfected with a myc-tagged full-length (FL-LNX2) and different truncated LNX2 (L2delPDZ234, L2delPDZ34, L2delPDZ4, L2PDZ1234, and L2delPDZ1234 into HEK293T cells. Coimmunoprecipitation was performed using the anti-myc antibody and probed with antibodies against Caspr4 (upper panel) and myc (lower panel) (G).