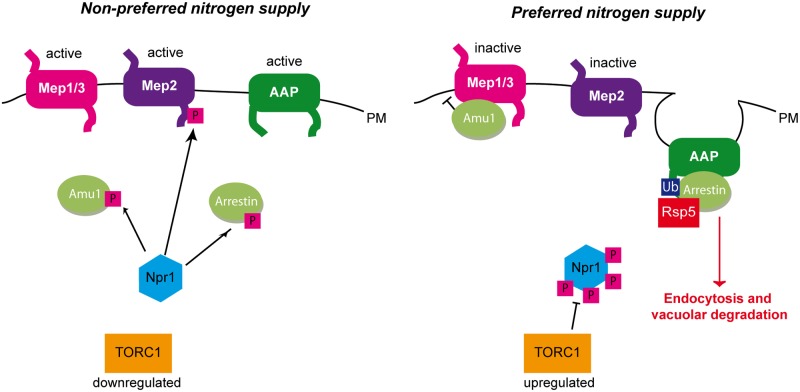
Fig 10. Three different strategies enable the TORC1-pathway to regulate plasma-membrane transport proteins.
Under non-preferred nitrogen supply, TORC1 is poorly active and Npr1 is hypophosphorylated and able to mediate the phosphorylation of its different targets. Npr1 mediates the phosphorylation of Amu1 which remains cytosolic, while Mep1 and Mep3 are kept active. Npr1 enables C-terminal phosphorylation of the Mep2 ammonium transport protein, thereby silencing an autoinhibitory domain and allowing Mep2 activity. Npr1 mediates phosphorylation of arrestin-like adaptors thereby protecting their amino acid permease (AAP) targets from endocytosis and vacuolar degradation. Under preferred nitrogen supply, TORC1 is upregulated, Npr1 is hyperphosphorylated and inhibited. In these conditions, Amu1 is dephosphorylated, and accumulates at the cell surface, physically interacts with Mep1 and Mep3, and mediates inhibition of ammonium transport. The non-phosphorylated autoinhibitory domain of Mep2 prevents the enhancer domain to activate the transport protein. Dephosphorylated arrestin-like adaptors recruit the Rsp5 ubiquitin-ligase to their AAP targets, which are ubiquitylated, endocytosed and degraded in the vacuole.