Figure 3. Human mAb prophylaxis and therapy against lethal CHIKV infection in Ifnar−/− mice.
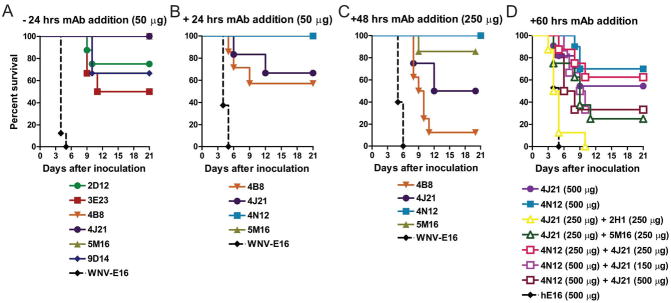
Mice were administered either 50 or 250 μg of indicated CHIKV-specific or control mAb by intraperitoneal injection 24 h before (A; n = 6 to 8 mice per mAb tested) or 24 h (B; n = 5 to 8 mice per mAb tested) or 48 h after (C; n = 7 to 10 mice per mAb tested) a lethal challenge of CHIKV (D) Mice were administered 150, 250 or 500 μg of indicated CHIKV-specific mAbs in combination by intraperitoneal injection 60 h after a lethal challenge of CHIKV (n = 6 to 13 mice per mAb combination tested). For monotherapy with 4J21, 4N12 or hE16 (negative control), a single dose of 500 μg was given (n = 10 to 17 mice per mAb tested). All data in this figure were pooled from at least two independent experiments. The following statistical analysis was performed using the Mantel-Cox log rank test: 4N12 versus 4J21, P = 0.39; 4N12 (500 μg) versus 4N12 (250 μg) + 4J21 (250 μg), P = 0.69; 4N12 (500 μg) versus 4N12 (500 μg) + 4J21 (150 μg), P = 0.13; 4N12 (500 μg) versus 4N12 (500 μg) + 4J21 (500 μg), P = 0.06. All Ab administrations with the exception of 4J21 (250 μg) + 2H1 (250 μg) differed significantly from the hE16 control (P < 0.002).
