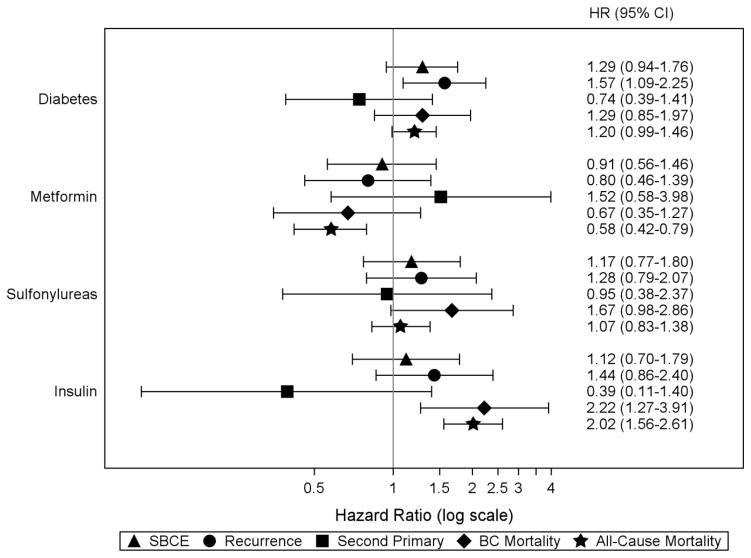
Figure 1.
Risk of SBCE, breast cancer-specific mortality, and all-cause mortality in relation to diabetes and diabetes medication classes* among all women in the COMBO cohort
Abbreviations: SBCE second breast cancer event (recurrence and second primary BC), BC breast cancer, HR hazard ratio, CI confidence interval
Note: all hazard ratios are adjusted for age at diagnosis (18–49, 50–59, 60–69, 70–79, 80+ years); diagnosis year (1990–1994, 1995–1999, 2000–2004, 2005–2008); AJCC stage (I, IIA, IIB); hormone receptor status (estrogen receptor [ER]−/progesterone receptor [PR] −, ER +/PR −, ER −/PR +, ER +/PR +, and ER and/or PR unknown); primary treatment for initial BC (mastectomy, breast conserving surgery with radiation, breast conserving surgery without radiation); endocrine therapy for the incident BC (yes/no, time-varying); body mass index (BMI) at diagnosis (<18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, 35+ kg/m2); smoking status at diagnosis (current, past, never/unknown); menopausal status at diagnosis (peri- or premenopausal, postmenopausal); Charlson co-morbidity score (0, 1, 2+, time-varying); statin use (yes/no, time-varying); prescription non-steroidal anti-inflammatory medication use, Cox-2 inhibitors, and aspirin (yes/no, time-varying); and receipt of screening mammogram in the 12 months prior to events (yes/no, time-varying).
* Hazard ratio for diabetes is from a model that did not adjust for diabetes medication use; hazard ratios for individual diabetes medication classes are from a separate model adjusting for all diabetes medication classes of interest (ever/never use, time-varying) and diabetes diagnosis (yes/no, time-varying)