Figure 1.
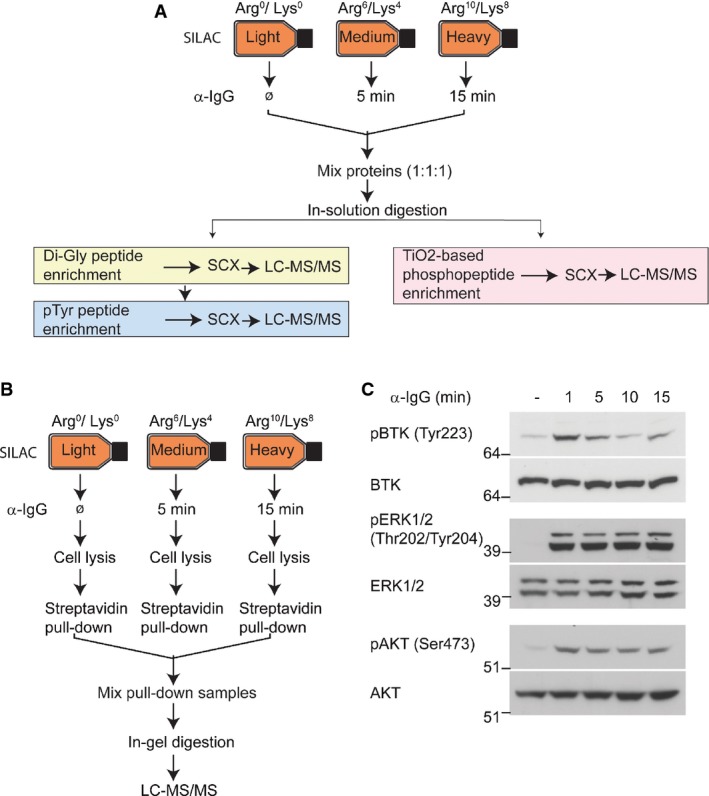
- Analysis of BCR-induced phosphorylation and ubiquitylation. A20 cells were isotopically labeled using the SILAC approach. Control “light” labeled cells were mock-treated, and “medium” and “heavy” labeled cells were stimulated with α-IgG F(ab′)2 for 5 and 15 min, respectively. Di-Gly-modified (ubiquitylated), tyrosine-phosphorylated peptides were enriched sequentially using di-Gly-lysine- and phosphotyrosine-specific antibodies. Phosphorylated peptides were separately enriched using TiO2-based chromatography. All samples were analyzed using high-resolution mass spectrometry.
- Strategy for analyzing the dynamics of BCR signalosomes. “Medium” and “heavy” SILAC-labeled A20 cells were stimulated with biotinylated α-IgG F(ab′)2 for 5 and 15 min, respectively. Control cells (labeled with “light” SILAC) were mock-treated. Proteins associated with biotinylated α-IgG F(ab′)2-bound BCR signalosomes were affinity-enriched using streptavidin, separated by SDS–PAGE, and analyzed by LC-MS/MS.
- Validation of BCR signaling activation. Stimulation of BCR signaling in A20 cells was confirmed using the indicated phosphorylation site-specific antibodies that recognize the activated forms of BTK, ERK1/2, and AKT kinases.
