Abstract
It has been believed that the peripheral lymphocytes in chickens proliferate by self-renewing amplification of the preimmune repertoire generated in bursa. We amplified rearranged immunoglobulin variable (V) region genes from the single germinal centers induced by immunization. The sequence analysis of these genes revealed that most were derived from distinct B-cell clones which expanded locally, generating somatic antibody mutants at a high rate. Somatic hypermutations included unlinked base changes and the linked base modifications interpreted as unidirectional transfer of sequences from V region pseudogenes. This finding demonstrates the ongoing post-bursal diversification of B-cells in splenic germinal centers by templated gene conversion as well as untemplated point mutations.
Full text
PDF


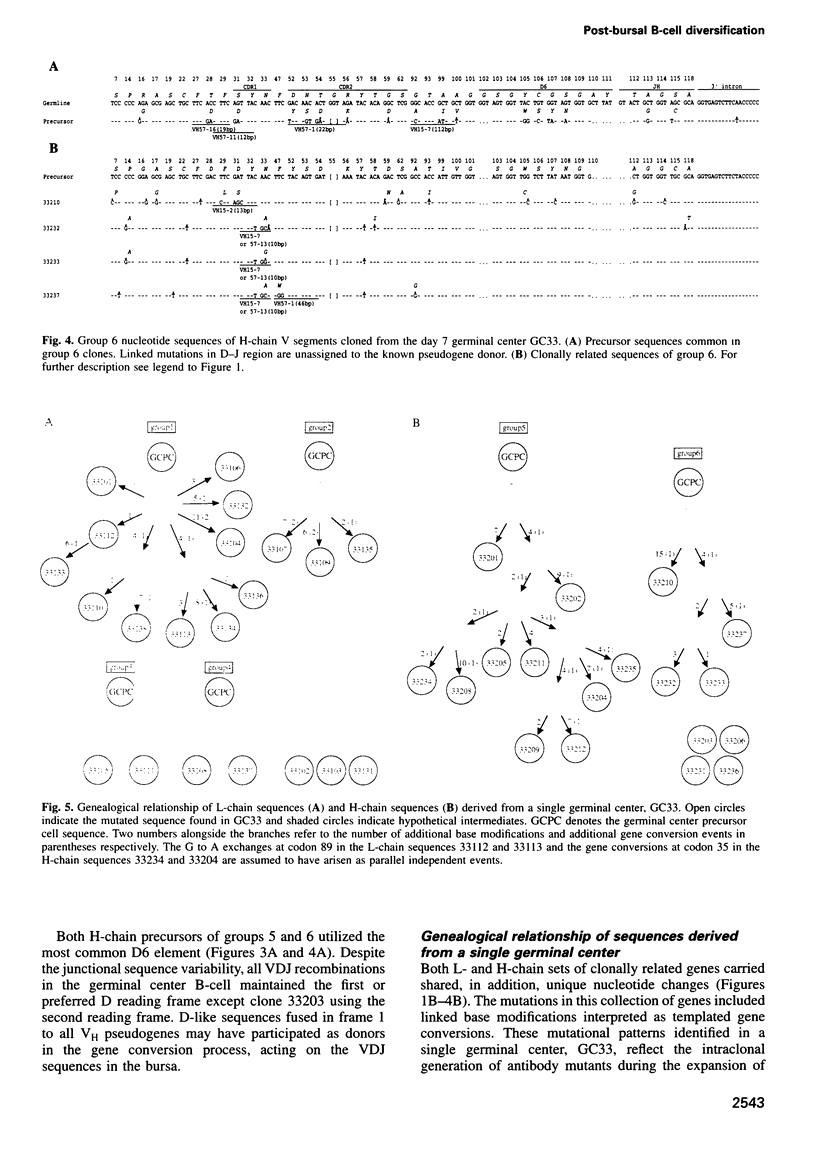



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berek C., Berger A., Apel M. Maturation of the immune response in germinal centers. Cell. 1991 Dec 20;67(6):1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- David V., Folk N. L., Maizels N. Germ line variable regions that match hypermutated sequences in genes encoding murine anti-hapten antibodies. Genetics. 1992 Nov;132(3):799–811. doi: 10.1093/genetics/132.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J. E., McHeyzer-Williams M. G., Lieber M. R. Analysis of individual immunoglobulin lambda light chain genes amplified from single cells is inconsistent with variable region gene conversion in germinal-center B cell somatic mutation. Eur J Immunol. 1994 Aug;24(8):1816–1822. doi: 10.1002/eji.1830240814. [DOI] [PubMed] [Google Scholar]
- Hraba T., Karakoz I., Madar J. Mechanisms of immunological tolerance in chickens. Folia Biol (Praha) 1977;23(6):439–440. [PubMed] [Google Scholar]
- Ivanyi J., Salerno A. Cellular mechanisms of escape from immunological tolerance. Immunology. 1972 Feb;22(2):247–257. [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kassir R., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991 May 1;173(5):1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G., Rajewsky K., Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991 Dec 5;354(6352):389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Kondo T., Arakawa H., Kitao H., Hirota Y., Yamagishi H. Signal joint of immunoglobulin V lambda 1-J lambda and novel joints of chimeric V pseudogenes on extrachromosomal circular DNA from chicken bursa. Eur J Immunol. 1993 Jan;23(1):245–249. doi: 10.1002/eji.1830230138. [DOI] [PubMed] [Google Scholar]
- Krawinkel U., Zoebelein G., Brüggemann M., Radbruch A., Rajewsky K. Recombination between antibody heavy chain variable-region genes: evidence for gene conversion. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4997–5001. doi: 10.1073/pnas.80.16.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese F. G., Timens W., Nieuwenhuis P. Germinal center reaction and B lymphocytes: morphology and function. Curr Top Pathol. 1990;84(Pt 1):103–148. doi: 10.1007/978-3-642-75519-4_5. [DOI] [PubMed] [Google Scholar]
- Kroese F. G., Wubbena A. S., Seijen H. G., Nieuwenhuis P. Germinal centers develop oligoclonally. Eur J Immunol. 1987 Jul;17(7):1069–1072. doi: 10.1002/eji.1830170726. [DOI] [PubMed] [Google Scholar]
- Küppers R., Zhao M., Hansmann M. L., Rajewsky K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 1993 Dec 15;12(13):4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebecque S. G., Gearhart P. J. Boundaries of somatic mutation in rearranged immunoglobulin genes: 5' boundary is near the promoter, and 3' boundary is approximately 1 kb from V(D)J gene. J Exp Med. 1990 Dec 1;172(6):1717–1727. doi: 10.1084/jem.172.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydyard P. M., Grossi C. E., Cooper M. D. Ontogeny of B cells in the chicken. I. Sequential development of clonal diversity in the bursa. J Exp Med. 1976 Jul 1;144(1):79–97. doi: 10.1084/jem.144.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C., Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986 Jun;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Mansikka A., Sandberg M., Lassila O., Toivanen P. Rearrangement of immunoglobulin light chain genes in the chicken occurs prior to colonization of the embryonic bursa of Fabricius. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9416–9420. doi: 10.1073/pnas.87.23.9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack W. T., Thompson C. B. Chicken IgL variable region gene conversions display pseudogene donor preference and 5' to 3' polarity. Genes Dev. 1990 Apr;4(4):548–558. doi: 10.1101/gad.4.4.548. [DOI] [PubMed] [Google Scholar]
- McCormack W. T., Tjoelker L. W., Carlson L. M., Petryniak B., Barth C. F., Humphries E. H., Thompson C. B. Chicken IgL gene rearrangement involves deletion of a circular episome and addition of single nonrandom nucleotides to both coding segments. Cell. 1989 Mar 10;56(5):785–791. doi: 10.1016/0092-8674(89)90683-1. [DOI] [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Grimal H., Weill J. C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987 Feb 13;48(3):379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Weill J. C. The chicken D locus and its contribution to the immunoglobulin heavy chain repertoire. Eur J Immunol. 1991 Nov;21(11):2661–2670. doi: 10.1002/eji.1830211104. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Dahan A., Anquez V., Weill J. C. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell. 1989 Oct 6;59(1):171–183. doi: 10.1016/0092-8674(89)90879-9. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Garcia C., Hein W. R., Weill J. C. Hypermutation generating the sheep immunoglobulin repertoire is an antigen-independent process. Cell. 1995 Jan 13;80(1):115–125. doi: 10.1016/0092-8674(95)90456-5. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Mackay C. R., Müller R. G., Weill J. C. Somatic generation of diversity in a mammalian primary lymphoid organ: the sheep ileal Peyer's patches. Cell. 1991 Mar 8;64(5):995–1005. doi: 10.1016/0092-8674(91)90323-q. [DOI] [PubMed] [Google Scholar]
- Smithyman A. M. A simple procedure for the isolation of germinal centres from chicken spleen. Dev Comp Immunol. 1977 Jul;1(3):263–270. doi: 10.1016/s0145-305x(77)80035-9. [DOI] [PubMed] [Google Scholar]
- Vainio O., Toivanen P., Granfors K., Pink J. R. Susceptibility to tolerance induction of bursal and peripheral B cells. Scand J Immunol. 1988 Nov;28(5):639–644. doi: 10.1111/j.1365-3083.1988.tb01496.x. [DOI] [PubMed] [Google Scholar]
- Weill J. C., Reynaud C. A., Lassila O., Pink J. R. Rearrangement of chicken immunoglobulin genes is not an ongoing process in the embryonic bursa of Fabricius. Proc Natl Acad Sci U S A. 1986 May;83(10):3336–3340. doi: 10.1073/pnas.83.10.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein P. D., Anderson A. O., Mage R. G. Rabbit IgH sequences in appendix germinal centers: VH diversification by gene conversion-like and hypermutation mechanisms. Immunity. 1994 Nov;1(8):647–659. doi: 10.1016/1074-7613(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Weiss U., Zoebelein R., Rajewsky K. Accumulation of somatic mutants in the B cell compartment after primary immunization with a T cell-dependent antigen. Eur J Immunol. 1992 Feb;22(2):511–517. doi: 10.1002/eji.1830220233. [DOI] [PubMed] [Google Scholar]
- White R. G., Henderson D. C., Eslami M. B., Neilsen K. H. Localization of a protein antigen in the chicken spleen. Effect of various manipulative procedures on the morphogenesis of the germinal centre. Immunology. 1975 Jan;28(1):1–21. [PMC free article] [PubMed] [Google Scholar]
- Ziegner M., Steinhauser G., Berek C. Development of antibody diversity in single germinal centers: selective expansion of high-affinity variants. Eur J Immunol. 1994 Oct;24(10):2393–2400. doi: 10.1002/eji.1830241020. [DOI] [PubMed] [Google Scholar]



