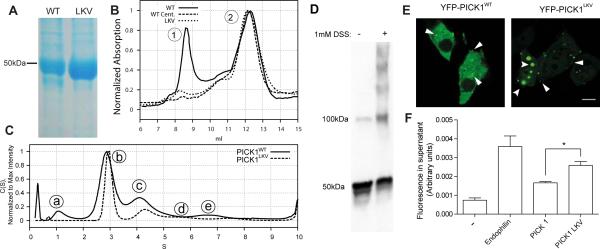
Figure 1. PICK1 exists in multiple oligomeric states and oligomerization is reduced in the self-binding mutant PICK1LKV.
(A) Representative SDS-PAGE (n=5) showing PICK1WT (left) and PICK1LKV (right). PICK1WT elutes at 46 kD (monomer). A weak putative degradation product is also seen. PICK1LKV displays only a band at 46 kD. (B) Size exclusion chromatography (SEC) of PICK1WT (solid line), ultracentrifuged PICK1WT (dashed line) and PICK1LKV (dotted line). PICK1WT (solid line) shows two major peaks. Peak 1 is removed by ultracentrifugation and is almost absent in PICK1LKV. (C) Analytical ultracentrifugation (AUC) of fractions corresponding to peak 2 in the SEC. PICK1WT (solid line) displays distinct peaks. PICK1LKV displays reduced size of peaks corresponding to larger sizes. (D) Representative western blot showing lysates from COS7 cells transiently expressing PICK1WT with or without pretreatment with the membrane permeable crosslinking agent DSS (n=3) (E) Representative confocal micrographs showing YFP-PICK1WT (left) and YFP-PICK1LKV (right) transiently expressed in COS7 cells (n=3). Arrows indicate clusters. Scale bar 10 μM. (F) Quantification of liposome vesiculation capacity of endophilin, PICK1WT and PICK1LKV in arbitrary units. Fluorescently labeled liposomes were incubated with indicated proteins before ultracentrifugation. Fluorescent lipids in the supernatant indicate scission from liposomes, which are pelleted (means ± sem, n=3, *p < 0.05). See also Figure S1.