Abstract
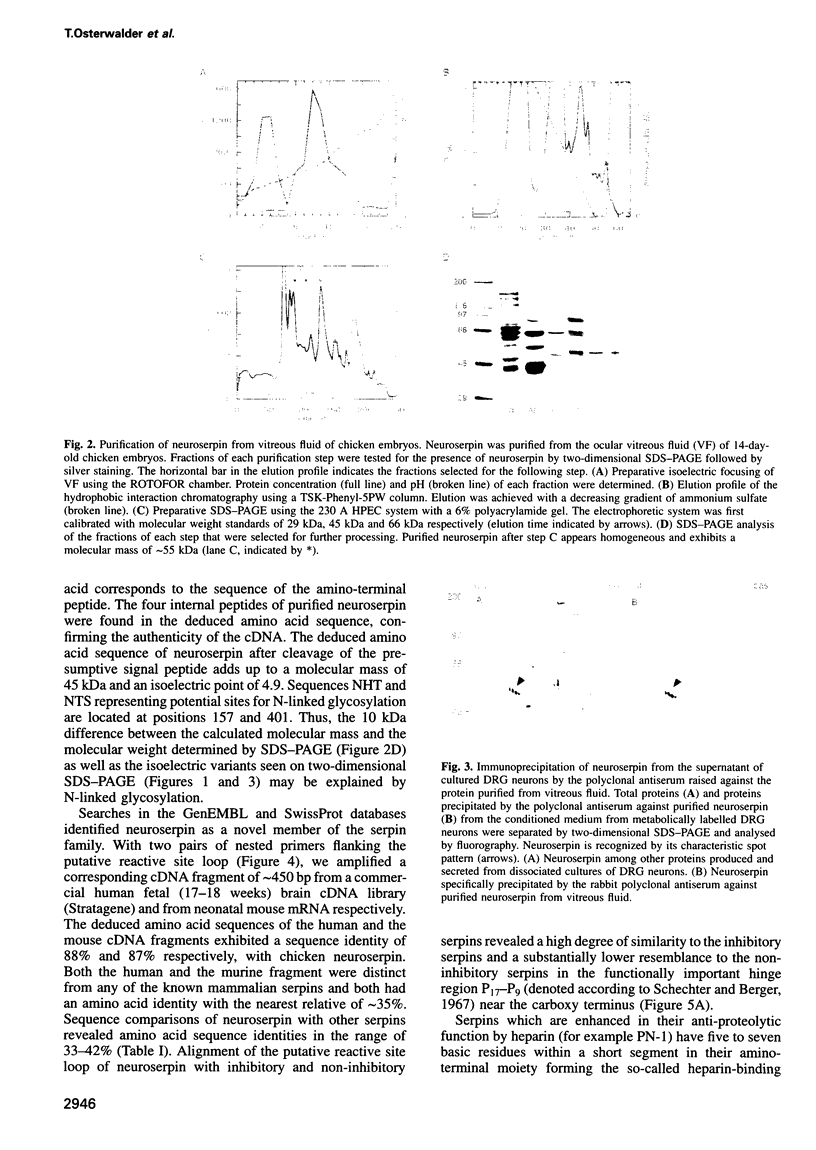
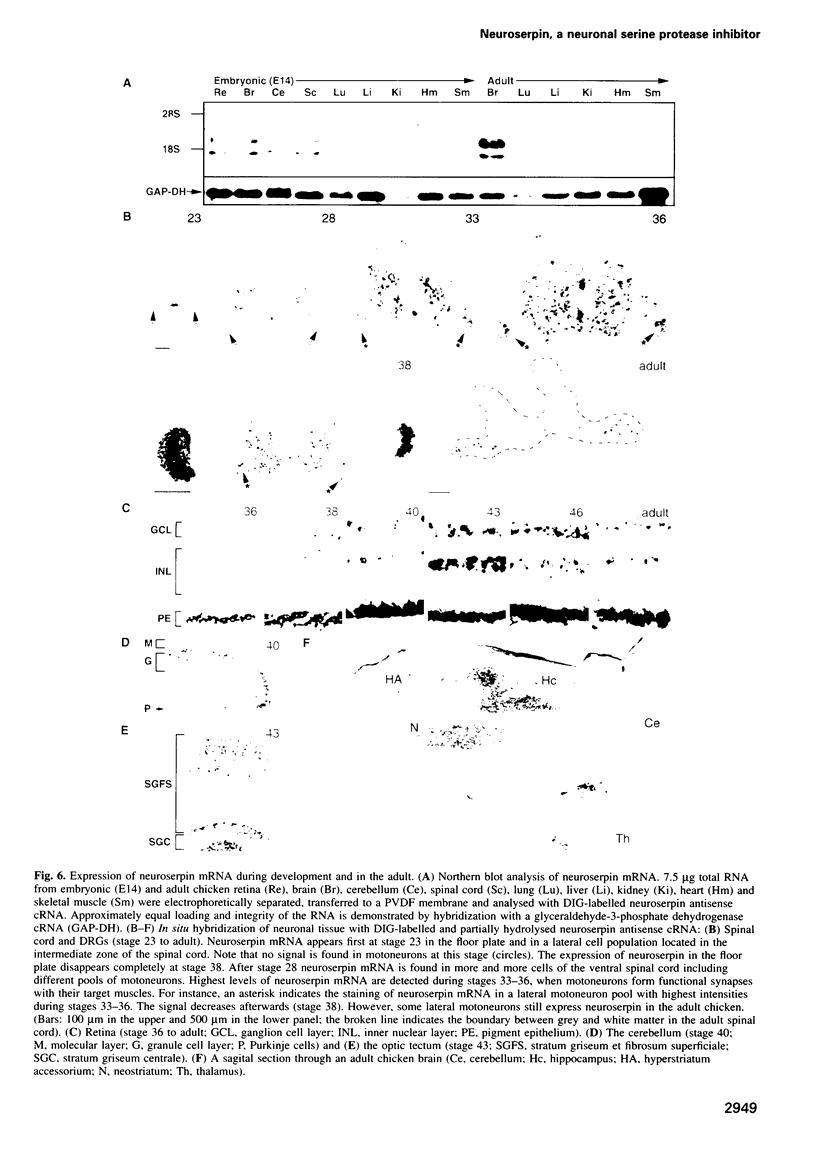
We have identified and chromatographically purified an axonally secreted glycoprotein of CNS and PNS neurons. Several peptides derived from it were microsequenced. Based on these sequences, a fragment of the corresponding cDNA was amplified and used as a probe to isolate a full length cDNA from a chicken brain cDNA library. Because the deduced amino acid sequence qualified the protein as a novel member of the serpin family of serine protease inhibitors, we called it neuroserpin. Analysis of the primary structural features further characterized neuroserpin as a heparin-independent, functional inhibitor of a trypsin-like serine protease. In situ hybridization revealed a predominantly neuronal expression during the late stages of neurogenesis and in the adult brain in regions which exhibit synaptic plasticity. Thus, neuroserpin might function as an axonally secreted regulator of the local extracellular proteolysis involved in the reorganization of the synaptic connectivity during development and synapse plasticity in the adult.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird J. L., Raper J. A. A serine proteinase involved in contact mediated repulsion of retinal growth cones by DRG neurites. J Neurosci. 1995 Oct;15(10):6605–6618. doi: 10.1523/JNEUROSCI.15-10-06605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Blackburn M. N., Smith R. L., Carson J., Sibley C. C. The heparin-binding site of antithrombin III. Identification of a critical tryptophan in the amino acid sequence. J Biol Chem. 1984 Jan 25;259(2):939–941. [PubMed] [Google Scholar]
- Blinder M. A., Tollefsen D. M. Site-directed mutagenesis of arginine 103 and lysine 185 in the proposed glycosaminoglycan-binding site of heparin cofactor II. J Biol Chem. 1990 Jan 5;265(1):286–291. [PubMed] [Google Scholar]
- Campenot R. B. Independent control of the local environment of somas and neurites. Methods Enzymol. 1979;58:302–307. doi: 10.1016/s0076-6879(79)58146-4. [DOI] [PubMed] [Google Scholar]
- Campenot R. B. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Connold A. L., Vrbová G. Neuromuscular contacts of expanded motor units in rat soleus muscles are rescued by leupeptin. Neuroscience. 1994 Nov;63(1):327–338. doi: 10.1016/0306-4522(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M., Cline H. T., Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Dent M. A., Sumi Y., Morris R. J., Seeley P. J. Urokinase-type plasminogen activator expression by neurons and oligodendrocytes during process outgrowth in developing rat brain. Eur J Neurosci. 1993 Jun 1;5(6):633–647. doi: 10.1111/j.1460-9568.1993.tb00529.x. [DOI] [PubMed] [Google Scholar]
- Deschepper C. F., Bigornia V., Berens M. E., Lapointe M. C. Production of thrombin and antithrombin III by brain and astroglial cell cultures. Brain Res Mol Brain Res. 1991 Oct;11(3-4):355–358. doi: 10.1016/0169-328x(91)90045-y. [DOI] [PubMed] [Google Scholar]
- Friedman K. D., Rosen N. L., Newman P. J., Montgomery R. R. Enzymatic amplification of specific cDNA inserts from lambda gt11 libraries. Nucleic Acids Res. 1988 Sep 12;16(17):8718–8718. doi: 10.1093/nar/16.17.8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettins P. Absence of large-scale conformational change upon limited proteolysis of ovalbumin, the prototypic serpin. J Biol Chem. 1989 Mar 5;264(7):3781–3785. [PubMed] [Google Scholar]
- Gettins P., Patston P. A., Schapira M. The role of conformational change in serpin structure and function. Bioessays. 1993 Jul;15(7):461–467. doi: 10.1002/bies.950150705. [DOI] [PubMed] [Google Scholar]
- Gloor S., Odink K., Guenther J., Nick H., Monard D. A glia-derived neurite promoting factor with protease inhibitory activity belongs to the protease nexins. Cell. 1986 Dec 5;47(5):687–693. doi: 10.1016/0092-8674(86)90511-8. [DOI] [PubMed] [Google Scholar]
- Goodman C. S., Shatz C. J. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993 Jan;72 (Suppl):77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Guenther J., Nick H., Monard D. A glia-derived neurite-promoting factor with protease inhibitory activity. EMBO J. 1985 Aug;4(8):1963–1966. doi: 10.1002/j.1460-2075.1985.tb03878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. H., Stratowa C., Rutter W. J. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987 Mar 24;26(6):1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- Hantaï D., Rao J. S., Kahler C., Festoff B. W. Decrease in plasminogen activator correlates with synapse elimination during neonatal development of mouse skeletal muscle. Proc Natl Acad Sci U S A. 1989 Jan;86(1):362–366. doi: 10.1073/pnas.86.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins P. C., Carrell R. W., Stone S. R. Effects of mutations in the hinge region of serpins. Biochemistry. 1993 Aug 3;32(30):7650–7657. doi: 10.1021/bi00081a008. [DOI] [PubMed] [Google Scholar]
- Huber R., Carrell R. W. Implications of the three-dimensional structure of alpha 1-antitrypsin for structure and function of serpins. Biochemistry. 1989 Nov 14;28(23):8951–8966. doi: 10.1021/bi00449a001. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Suzuki K. Separation of peptides dissolved in a sodium dodecyl sulfate solution by reversed-phase liquid chromatography: removal of sodium dodecyl sulfate from peptides using an ion-exchange precolumn. Anal Biochem. 1990 May 1;186(2):264–268. doi: 10.1016/0003-2697(90)90077-m. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. W. Plasminogen activator release at the neuronal growth cone. Science. 1981 Sep 25;213(4515):1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. W. Plasminogen activator secretion by granule neurons in cultures of developing cerebellum. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7810–7814. doi: 10.1073/pnas.78.12.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail J. H., Cowan W. M. The development of the chick optic tectum. II. Autoradiographic studies. Brain Res. 1971 May 21;28(3):421–441. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Strandberg L., Ericson J., Ny T. Structure-function studies of the SERPIN plasminogen activator inhibitor type 1. Analysis of chimeric strained loop mutants. J Biol Chem. 1990 Nov 25;265(33):20293–20301. [PubMed] [Google Scholar]
- Liu Y., Fields R. D., Festoff B. W., Nelson P. G. Proteolytic action of thrombin is required for electrical activity-dependent synapse reduction. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10300–10304. doi: 10.1073/pnas.91.22.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier R., Spreyer P., Ortmann R., Harel A., Monard D. Induction of glia-derived nexin after lesion of a peripheral nerve. Nature. 1989 Nov 30;342(6249):548–550. doi: 10.1038/342548a0. [DOI] [PubMed] [Google Scholar]
- Meiri N., Masos T., Rosenblum K., Miskin R., Dudai Y. Overexpression of urokinase-type plasminogen activator in transgenic mice is correlated with impaired learning. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3196–3200. doi: 10.1073/pnas.91.8.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mey J., Thanos S. Development of the visual system of the chick--a review. J Hirnforsch. 1992;33(6):673–702. [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Nick H., Hofsteenge J., Shaw E., Rovelli G., Monard D. Functional sites of glia-derived nexin (GDN): importance of the site reacting with the protease. Biochemistry. 1990 Mar 6;29(9):2417–2421. doi: 10.1021/bi00461a027. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Shneiderman A., Shimizu I., Yaginuma H. Onset and development of intersegmental projections in the chick embryo spinal cord. J Comp Neurol. 1988 Sep 8;275(2):159–180. doi: 10.1002/cne.902750202. [DOI] [PubMed] [Google Scholar]
- Qian Z., Gilbert M. E., Colicos M. A., Kandel E. R., Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993 Feb 4;361(6411):453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Rao J. S., Chen M., Festoff B. W. Plasminogen activator inhibitor 1, the primary regulator of fibrinolysis, in normal human cerebrospinal fluid. J Neurosci Res. 1993 Feb 15;34(3):340–345. doi: 10.1002/jnr.490340311. [DOI] [PubMed] [Google Scholar]
- Rayford A., Rao J. S., Festoff B. W. Characterization of the serpin, alpha 1-antichymotrypsin, in normal human cerebrospinal fluid. J Neurochem. 1992 Jan;58(1):88–94. doi: 10.1111/j.1471-4159.1992.tb09281.x. [DOI] [PubMed] [Google Scholar]
- Ruegg M. A., Stoeckli E. T., Kuhn T. B., Heller M., Zuellig R., Sonderegger P. Purification of axonin-1, a protein that is secreted from axons during neurogenesis. EMBO J. 1989 Jan;8(1):55–63. doi: 10.1002/j.1460-2075.1989.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeren-Wiemers N., Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993 Dec;100(6):431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Schulze A. J., Huber R., Bode W., Engh R. A. Structural aspects of serpin inhibition. FEBS Lett. 1994 May 16;344(2-3):117–124. doi: 10.1016/0014-5793(94)00369-6. [DOI] [PubMed] [Google Scholar]
- Sheets M. D., Ogg S. C., Wickens M. P. Point mutations in AAUAAA and the poly (A) addition site: effects on the accuracy and efficiency of cleavage and polyadenylation in vitro. Nucleic Acids Res. 1990 Oct 11;18(19):5799–5805. doi: 10.1093/nar/18.19.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. M., Lawrence D. A., Yang A. Y., Vandenberg E. T., Paielli D., Olson S. T., Shore J. D., Ginsburg D. Saturation mutagenesis of the plasminogen activator inhibitor-1 reactive center. J Biol Chem. 1992 Apr 15;267(11):7588–7595. [PubMed] [Google Scholar]
- Sonderegger P., Fishman M. C., Bokoum M., Bauer H. C., Neale E. A., Nelson P. G. A few axonal proteins distinguish ventral spinal cord neurons from dorsal root ganglion neurons. J Cell Biol. 1984 Jan;98(1):364–368. doi: 10.1083/jcb.98.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger P., Fishman M. C., Bokoum M., Bauer H. C., Nelson P. G. Axonal proteins of presynaptic neurons during synaptogenesis. Science. 1983 Sep 23;221(4617):1294–1297. doi: 10.1126/science.6612344. [DOI] [PubMed] [Google Scholar]
- Sonderegger P., Lemkin P. F., Lipkin L. E., Nelson P. G. Differential modulation of the expression of axonal proteins by non-neuronal cells of the peripheral and central nervous system. EMBO J. 1985 Jun;4(6):1395–1401. doi: 10.1002/j.1460-2075.1985.tb03792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein P. E., Leslie A. G., Finch J. T., Turnell W. G., McLaughlin P. J., Carrell R. W. Crystal structure of ovalbumin as a model for the reactive centre of serpins. Nature. 1990 Sep 6;347(6288):99–102. doi: 10.1038/347099a0. [DOI] [PubMed] [Google Scholar]
- Stoeckli E. T., Landmesser L. T. Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron. 1995 Jun;14(6):1165–1179. doi: 10.1016/0896-6273(95)90264-3. [DOI] [PubMed] [Google Scholar]
- Stoeckli E. T., Lemkin P. F., Kuhn T. B., Ruegg M. A., Heller M., Sonderegger P. Identification of proteins secreted from axons of embryonic dorsal-root-ganglia neurons. Eur J Biochem. 1989 Mar 15;180(2):249–258. doi: 10.1111/j.1432-1033.1989.tb14640.x. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Brown-Luedi M. L., Rovelli G., Guidolin A., McGlynn E., Monard D. Localization of the heparin-binding site of glia-derived nexin/protease nexin-1 by site-directed mutagenesis. Biochemistry. 1994 Jun 21;33(24):7731–7735. doi: 10.1021/bi00190a028. [DOI] [PubMed] [Google Scholar]
- Sumi Y., Dent M. A., Owen D. E., Seeley P. J., Morris R. J. The expression of tissue and urokinase-type plasminogen activators in neural development suggests different modes of proteolytic involvement in neuronal growth. Development. 1992 Nov;116(3):625–637. doi: 10.1242/dev.116.3.625. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J. Environmentally regulated expression of soluble extracellular proteins of sympathetic neurons. J Biol Chem. 1981 Apr 25;256(8):4063–4070. [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Udin S. B., Fawcett J. W. Formation of topographic maps. Annu Rev Neurosci. 1988;11:289–327. doi: 10.1146/annurev.ne.11.030188.001445. [DOI] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Wright H. T., Qian H. X., Huber R. Crystal structure of plakalbumin, a proteolytically nicked form of ovalbumin. Its relationship to the structure of cleaved alpha-1-proteinase inhibitor. J Mol Biol. 1990 Jun 5;213(3):513–528. doi: 10.1016/s0022-2836(05)80212-8. [DOI] [PubMed] [Google Scholar]
- Zuellig R. A., Rader C., Schroeder A., Kalousek M. B., Von Bohlen und Halbach F., Osterwalder T., Inan C., Stoeckli E. T., Affolter H. U., Fritz A. The axonally secreted cell adhesion molecule, axonin-1. Primary structure, immunoglobulin-like and fibronectin-type-III-like domains and glycosyl-phosphatidylinositol anchorage. Eur J Biochem. 1992 Mar 1;204(2):453–463. doi: 10.1111/j.1432-1033.1992.tb16655.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]