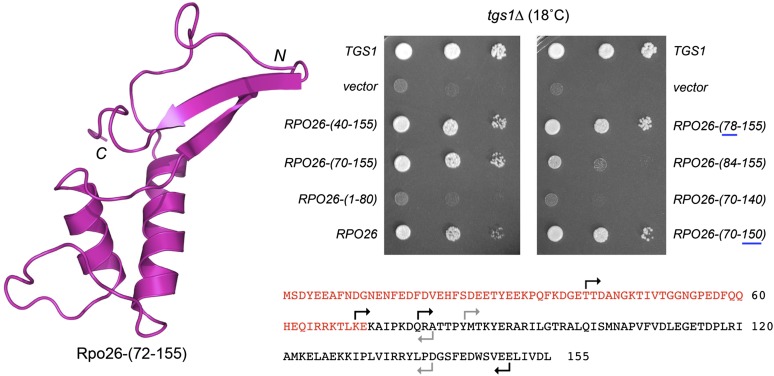
Figure 5.
N- and C-terminal truncations of Rpo26 delineate a minimal functional domain for tgs1∆ suppression. (Left) Tertiary structure of Rpo26-(72-155), from the yeast Pol II crystal structure (pdb 1I3Q), with the N and C termini indicated. (Bottom right) The amino acid sequence of yeast Rpo26. The C-terminal segment visualized in the Pol II crystal structure is in black font; the disordered N-terminal segment is in red font. The margins of the N- and C-terminal truncations are denoted by forward and reverse arrows. For the N-terminal deletions, the arrows specify the residues that were mutated to methionine to initiate the truncated proteins. Black arrows denote the truncations that allow the mutants to restore tgs1Δ growth at 18°, whereas the gray arrows denote the truncations that disable tgs1∆ suppression. (Top right) tgs1Δ cells were transformed with a CEN LEU2 plasmid bearing wild-type TGS1 (positive control), an empty 2-μ LEU2 TPI1 vector (negative control), or 2-μ LEU2 TPI1-RPO26 plasmids bearing wild-type RPO26 or the indicated truncation mutants. Leu+ transformants were selected at 30° and then tested for growth at 18° by spotting serial 10-fold dilutions of liquid cultures (grown at 30° in SD-Leu medium) on SD-Leu agar plates. The plates were photographed after incubation for 7 d at 18°.