FIGURE 2.
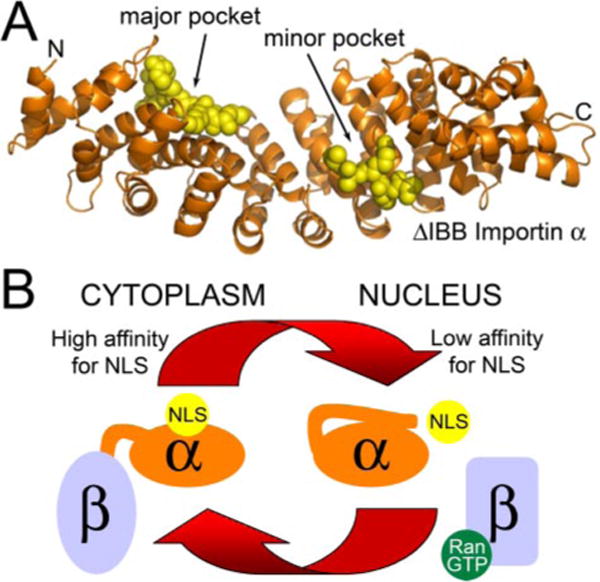
A, the importin α/cNLS interaction. cNLS peptides bound to the major and minor binding pockets of S. cerevisiae importin α lacking the IBB domain (Protein Data Bank entry 1BK6) (50). Importin β(amino acids 88–530) is shown in orange. Two SV40 peptides bound to the major and minor binding pockets are shown in yellow. B, regulation of cNLS cargo binding to importin α. In the cytoplasm, importin β is bound to the flexible autoinhibitory domain of importin α, sequestering it from the NLS-binding pocket and allowing cNLSs to bind to importin α with high affinity. Once in the nucleus, RanGTP binding to importin β causes a conformational change that releases the autoinhibitory domain, which can then compete for binding to the NLS-binding pocket. This competition contributes to a low affinity of the cNLS for importin α in the nucleus, facilitating cNLS cargo delivery.
