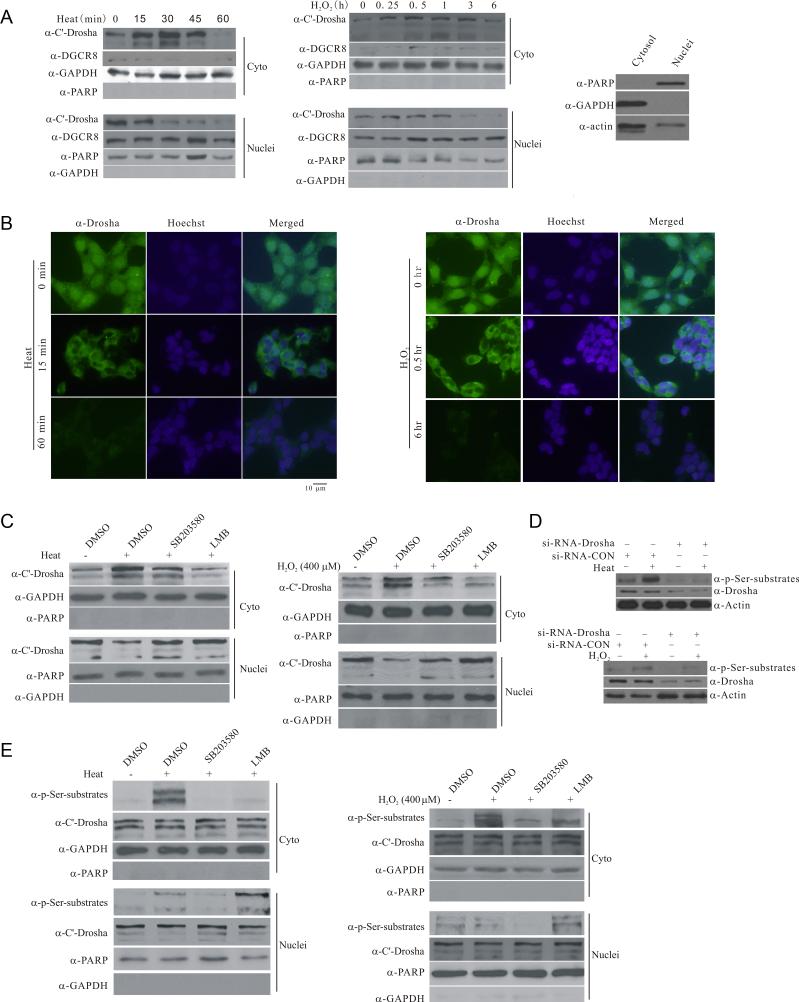
Figure 3.
Stress-induced phosphorylation-dependent nuclear export of Drosha. (A) Stress-induced changes in the subcellular distribution of endogenous Drosha. HEK293 cells were exposed to heat (45°C) or H2O2 (400 μM) for the indicated time. Cytoplasmic and nuclear fractions were blotted for Drosha, cytoplasmic marker GAPDH or nuclear marker PARP1 (PARP). Right panel shows the cross contamination of cytoplasmic and nuclear fractions yielded by the Sigma Nuclei EZ Prep Kit (Nuc-01). (B) Stress-induced changes in the subcellular distribution of endogenous Drosha by immunocytochemistry. HEK297 cells were exposed to heat (45°C) or H2O2 (400 μM) for the indicated time. The cells were stained with anti-Drosha antibody or Hoechst33324. All Drosha images were acquired with 200 ms exposure time and Hoechst image 10 ms exposure time. (C) Stress-induced p38 MAPK dependent accumulation of endogenous Drosha in the cytoplasm. HEK293T cells were treated with SB203580 (20 μM) or leptomycin B (LMB, 5 ng/ml) for 30 min and exposed to heat (45°C, 30 min) or H2O2 (400 μM, 30 min). The levels of Drosha in the cytoplasmic and nuclear fractions were determined. (D) Recognition of stress-induced Drosha phosphorylation by anti-phospho Ser antibody. HEK293 cells were transfected with si-RNA-Drosha or si-RNA-control for 72 h and exposed to heat (45°C) or H2O2 (400 μM) for 15 min. The proteins were collected for western blot with anti-phospho Ser antibody. (E) Stress-induced nuclear export of phosphorylated Drosha. HEK293 cells were treated as in (C) with 15 min stress. See also Figure S3.