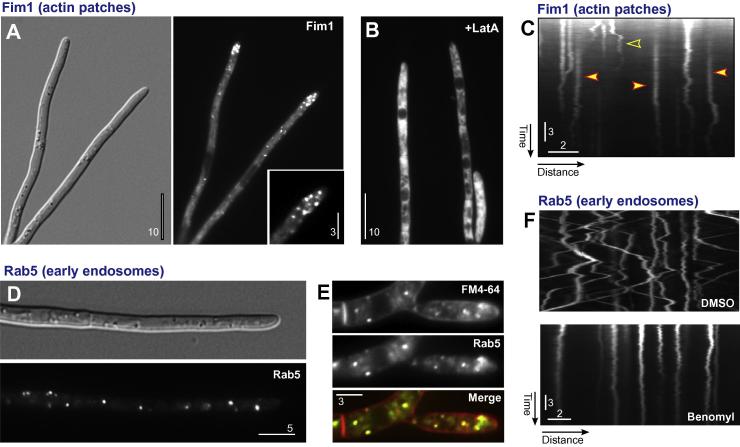
Fig. 3.
Compartments of the early endocytic pathway in Z. tritici. (A) Hyphal cells expressing Fim1-eGFP, after ectopic integration of pHFim1eGFP. The actin-binding protein concentrates in patches at the growth region (see inset). This localization is typical for actin patches in fungi. Bars represent 10 μm and 3 μm. (B) Fim1-eGFP in the presence of the actin-disrupting drug Latrunculin A (incubated at 10 μM for 30 min). Actin-patches disappeared and the fluorescent fusion protein locates in the cytoplasm. Bar represents 10 μm. (C) Kymograph showing motility of Fim1-eGFP signals in Z. tritici. Signals remain stationary, but start random lateral movement before they disappear (open arrowhead). Note that several Fim1-eGFP signals appear during the course of observation (filled arrowheads). This motility behavior is consistent with that of fungal actin patches. Bars represent 3 s and 2 μm. (D) Localization of the early endosome marker protein eGFP-Rab5, expressed after ectopic integration of pHeGFPRab5, in a hyphal cell of Z. tritici. Bar represents 5 μm. (E) Co-visualization of eGFP-Rab5 (green in overlay) and the endocytic marker dye FM4-64 (red in overlay) in Z. tritici. The dye is concentrated in the plasma membrane, from where it is taken up into early endosomes. These organelles co-localize with eGFP-Rab5 (yellow) in overlay. This confirms that the marker labels an early endocytic compartment. Bar represents 3 μm. (F) Kymograph showing motility of eGFP-Rab5-labelled early endosomes. Moving signals are represented by diagonal lines, whereas stationary signals are provide vertical lines. Note that treatment with the microtubule-inhibitor benomyl (300 μM, 45 min) abolishes motility. This is consistent with a microtubule-based transport of early endosomes, reported in other fungal systems. Bars represent 3 s and 2 μm.